

Efficacy of TAFINLAR® (dabrafenib) + MEKINIST® (trametinib)
TAFINLAR in combination with MEKINIST is indicated in adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAF V600 mutation.1,2
Common adverse events include:
TAFINLAR + MEKINIST: The most common adverse reactions (incidence ≥ 20%) for dabrafenib in combination with trametinib were pyrexia, fatigue, nausea, chills, headache, diarrhoea, vomiting, arthralgia and rash1,2
TAFINLAR: The most common adverse reactions (incidence >15%) reported with dabrafenib were hyperkeratosis, headache, pyrexia, arthralgia, fatigue, nausea, papilloma, alopecia, rash and vomiting1
MEKINIST: The most common adverse reactions (incidence ≥20%) for trametinib were rash, diarrhoea, fatigue, oedema peripheral, nausea and dermatitis acneiform2
For more safety information on TAFINLAR and MEKINIST, click here.
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
Durable overall survival (OS) rate in advanced NSCLC
In a 5-year follow-up study of treatment-naïve and pretreated patients, with a median follow-up of 16.3 and 16.6 months, respectively, the estimated median OS rates were 17.3 months (95% CI: 12.3–40.2) and 18.2 months (95% CI: 14.3–28.6).3
TAFINLAR + MEKINIST is a proven, first-line treatment option for eligible patients with BRAF V600-mutated NSCLC1–4
How effective is TAFLINAR + MEKINIST in newly diagnosed patients with BRAF V600E-mutated, advanced NSCLC?
Study design
The efficacy and safety profile of TAFLINAR + MEKINIST was assessed in 171 patients with BRAF V600E mutation-positive NSCLC in a Phase II, multicohort, multicentre, non-randomised, open-label study (NCT01336634). Patients were sequentially enrolled into 3 different cohorts based on the number of prior lines of systemic treatment for metastatic disease.1,2,5,6
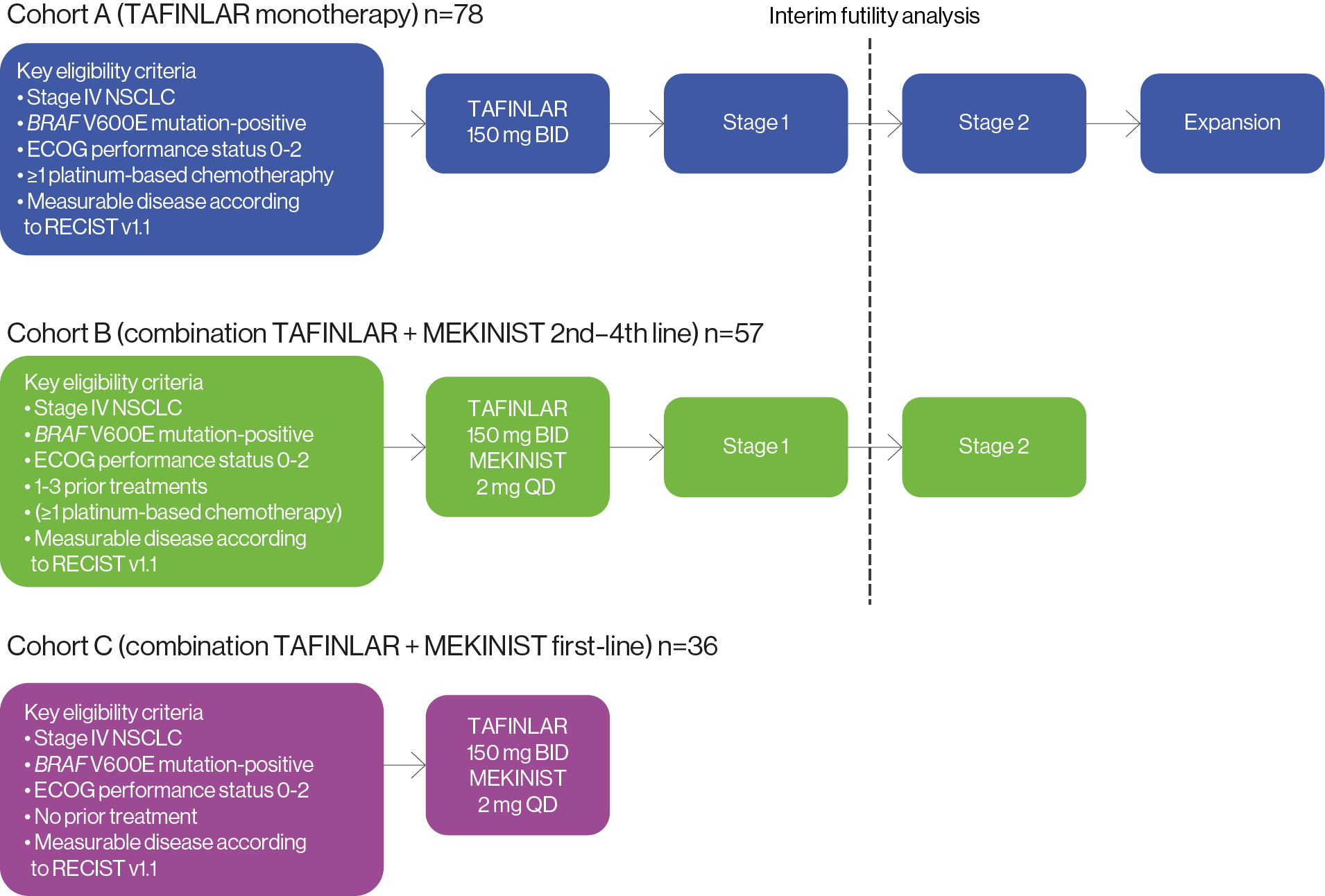
Study endpoints: | |
Primary endpoint | Investigator-assessed ORR* |
Secondary endpoints | PFS, OS, DOR, safety, tolerability and pharmacokinetics |
Adapted from Planchard D, et al. ASCO virtual meeting, May 29–31, 2020. Poster6
*Defined as the percentage of patients who achieved a confirmed complete response or partial response per Response Evaluation Criteria In Solid Tumours version 1.1 (RECIST v1.1).
Inclusion/exclusion criteria
Baseline demographics
Baseline characteristics of pre-treated (cohort B) and treatment-naïve (cohort C) patients3
Baseline characteristic | Pre-treated (cohort B) | Treatment-naïve (cohort C) |
Age (years) | ||
Median (range) | 64.0 (41–88) | 67.0 (44–91) |
Age group (years), n (%) | ||
≥18 to <65 | 29 (51) | 14 (39) |
≥65 | 28 (49) | 22 (61) |
Sex, n (%) | ||
Female | 28 (49) | 22 (61) |
ECOG performance status, n (%) | ||
0 | 17 (30) | 13 (36) |
1 | 35 (61) | 22 (61) |
2 | 5 (9) | 1 (3) |
Smoking history, n (%) | ||
Never smoked | 16 (28) | 10 (28) |
Current smoker | 6 (11) | 5 (14) |
Former smoker | 35 (61) | 21 (58) |
Efficacy outcomes in first and second-line patients
ORR, DOR, PFS and OS for pre-treated (cohort B) and treatment-naïve (cohort C) patients3
Endpoint | Pre-treated (cohort B) | Treatment-naïve (cohort C) |
ORR, % (CR+PR) | 68.4 (39) | 63.9 (23) |
mDOR, months (95% CI) | 9.8 (6.9–18.3) | 10.2 (8.3–15.2) |
mOS, months (95% CI) | 18.2 (14.3–28.6) | 17.3 (12.3–40.2) |
5-year survival rates, % | 19 | 22 |
1L, first-line; BID, twice daily; BRAF V600E, mutation of the BRAF gene at valine (V) 600 to glutamate (E); CI, confidence interval; CR, complete response; DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; MEK, mitogen-activated extracellular signal-regulated kinase; NE, not estimable; NSCLC, non-small cell lung cancer; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; QD, once daily; RECIST, Response Evaluation Criteria In Solid Tumours; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
Planchard D, et al. J Thorac Oncol 2022;17:103–115.
Planchard D, et al. Lancet Oncol 2017;18:1307–1316.
Clinicaltrials.gov. NCT01336634 Available at: https://clinicaltrials.gov/ct2/show/record/NCT01336634?view=record. [Accessed April 2025].
Planchard D, et al. ASCO virtual meeting, May 29–31, 2020. Poster.
UK | April 2025 | FA-11217989
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.




