
Voltaren 75mg/3ml ampoules
Indication
Voltaren® 75mg/3ml ampoules contains the sodium salt of diclofenac, a non-steroidal agent with pronounced antirheumatic, anti-inflammatory, analgesic and antipyretic activity1
In post-traumatic and post-operative inflammatory conditions, Voltaren® rapidly relieves both spontaneous pain and pain on movement, and reduces inflammatory swelling and wound oedema2
Dosage
Image

| The dose is generally one 75 mg ampoule daily1 |
Image

| In severe cases (e.g., colic),the daily dose canexceptionally be increased to two 75 mgampoules, separated by an interval of a fewhours (one into each buttock}.1 |
Efficacy
During postoperative period, Patients who achieved pain relief in the Voltaren® IM (n = 55) were significantly more than those who did not achieve pain relief ( n = 5 ; p < 0.05 ).2
Pain during postoperative period (60 minutes) in the Voltaren® IM group2
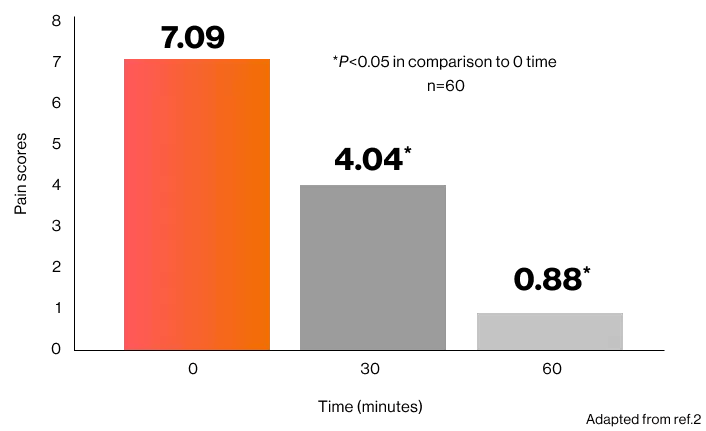
Pharmacokinetics
Peak Plasma Concentrations are reached after about 20 minutes.1
Time required by Voltaren® IM injection to reach mean peak plasma concertation1
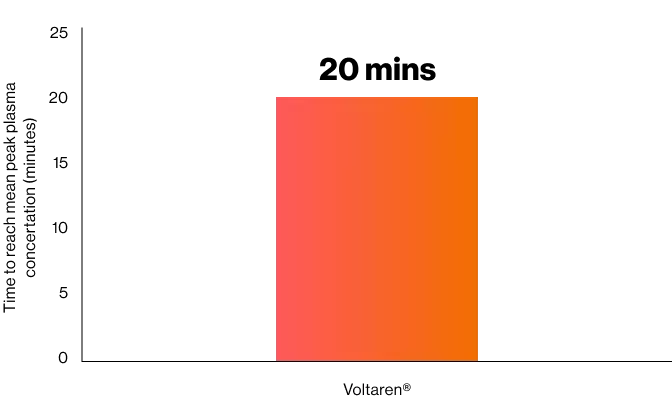

Image

| Diclofenac should therefore not be used by women who are breast-feeding.If treatment is essential, the infant should be switched to bottle feeding1 |
For Voltaren® Amp. Abbreviated prescribing information
For Voltaren® Amp. Abbreviated prescribing information
References
Voltaren®ampoules Egyptian drug authority leaflet approval date. Approved on 10/02/2025
Al-Waili NS. Efficacy and safety of repeated postoperative administration of intramuscular diclofenac sodium in the treatment of post-cesarean section pain: a double-blind study. Archives of Medical Research. 2001 Mar 1;32(2):148-54
Approved by Egyptian Drug Authority: HF0082OA4731/092025. Invalidation date: 01/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4731/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
