
Cataflam® 75mg/3mL solution for injection
Indication
Pronounced analgesic effect in moderate and severe pain.1
Cataflam® 75mg/3mL (diclofenac potassium) ampoule is indicated in short-term treatment in the following acute conditions:1
Image

| Post-traumatic pain, inflammation, and swelling e.g., due to sprains |
Image

| Postoperative pain, inflammation, and swelling e.g., following dental pain or orthopedic surgery. |
Dosage
Image

| The dose is generally one 75 mg ampoule daily.1 |
Image

| In severe cases (e.g., colic), the daily dose can be increased to two injections of 75 mg.1 |
Pharmacokinetics
- Cataflam® 75 mg/3 mL takes only 10 minutes to reach 80% of the mean maximum plasma concentration.1
- In the presence of inflammation, it relieves both spontaneous pain and pain on Movement.1
Time to reach 80% of Cmax

Efficacy
Analgesic drug combination of paracetamol-tramadol and paracetamol-diclofenac reduce total tramadol consumption and prolongs time to first analgesic request compared to paracetamol before surgical intervention alone in patients undergoing laparotomy surgery.2
Time to first analgesic request in minutes and total analgesia consumption over 24 hours 2
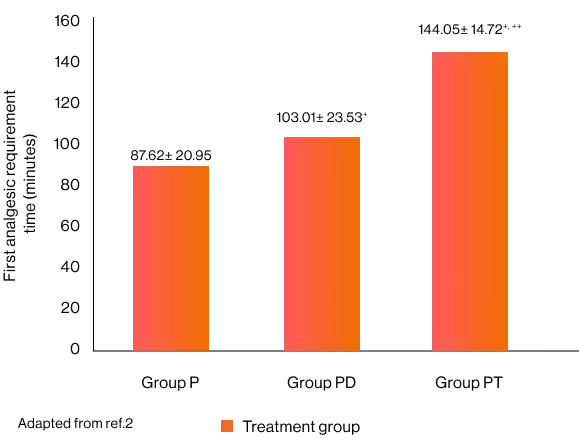
+p < 0.05 compared to Group P, ++ p < 0.05 compared to Group PD
The time to first analgesia requirement in the paracetamol group was significantly shorter than in the paracetamol-diclofenac group and paracetamol-tramadol groups.
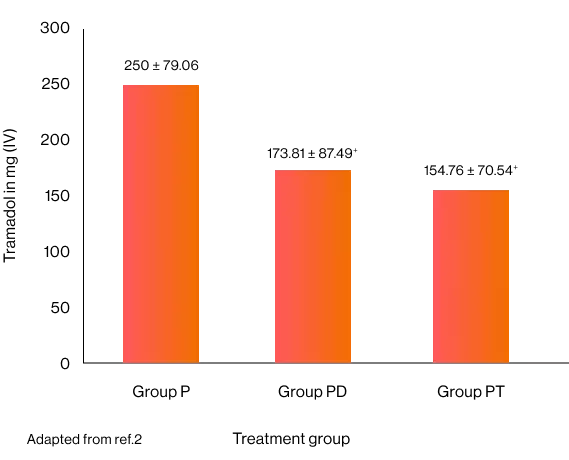
+p < 0.05 compared to Group P, ++ p < 0.05 compared to Group PD
Post hoc analysis of total tramadol consumed in 24 h showed significantly higher in paracetamol group compared to paracetamol-diclofenac group and paracetamol-tramadol group.
For Cataflam® 75 mg/3 mL Abbreviated prescribing information
For Cataflam® 75 mg/3 mL Abbreviated prescribing information
References
Cataflam® (diclofenac potassium) 75 mg/3 mL solution for injection. Egyptian Drug Authority approved insert leaflet, Approval date: 23 /9/2024.
Aweke Z, Seyoum F, Shitemaw T, Doba DN. Comparison of preemptive paracetamol, paracetamol-diclofenac & paracetamol-tramadol combination on postoperative pain after elective abdominal surgery under general anesthesia, Ethiopia: a randomized control trial study, 2018. BMC anesthesiology. 2020 Dec;20:1-9.
Approved by Egyptian Drug Authority: HF0082OA4731/092025. Invalidation date: 01/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0082OA4731/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
