
Difficult To Treat
Long-lasting skin clearance—even in nail psoriasis
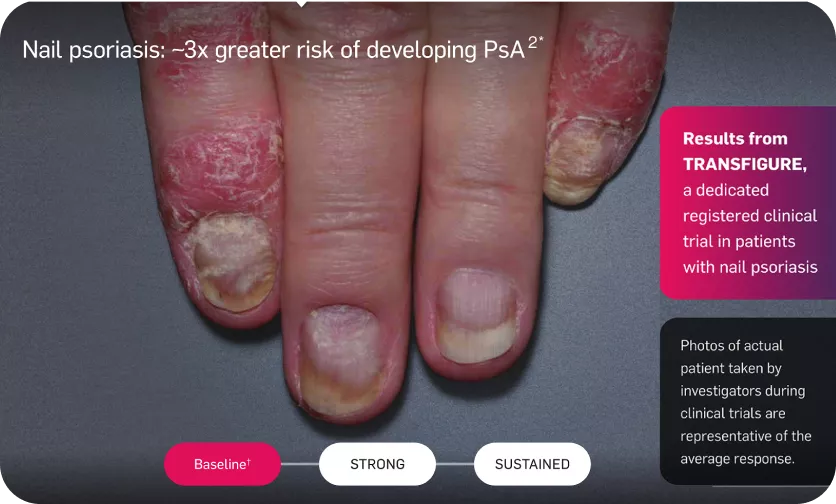
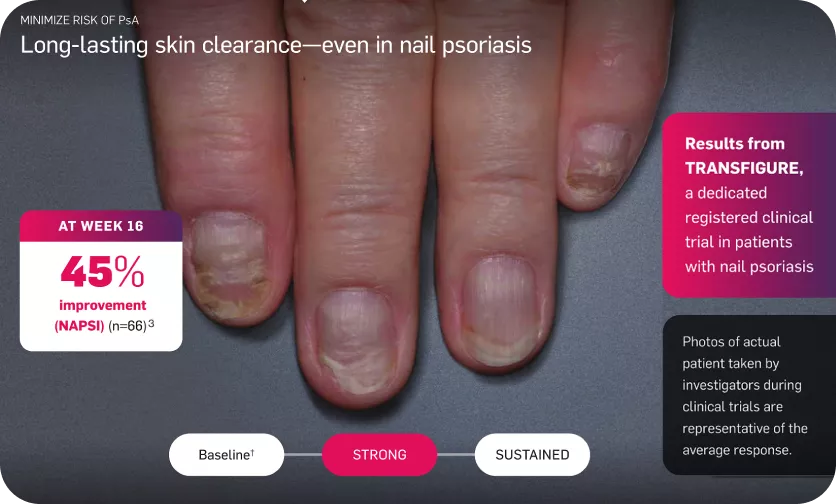
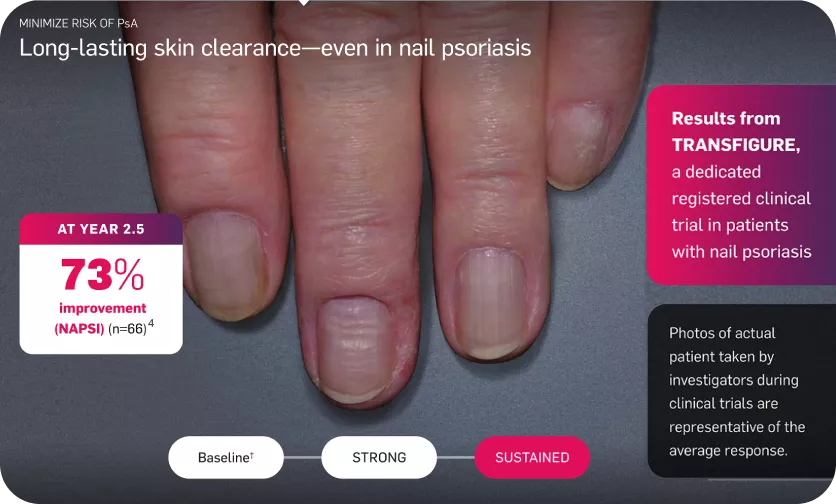
Adapted from reference 3
The TRANSFIGURE study was conducted to evaluate the efficacy and safety of Cosentyx in patients with moderate-to-severe nail psoriasis.
Randomization was managed via a central interactive randomization system and ensured that an equal number of patients were allocated to Cosentyx 300 mg, Cosentyx150 mg or placebo, stratified by body weight (< 90 kg or 90 kg). At week 16, all patients receiving placebo were rerandomized 1:1 to receive either 300 mg or 150 mg Cosentyx.
Patients received subcutaneous treatments of identical appearance once a week for 5 weeks (at baseline and weeks 1, 2, 3 and 4), followed by dosing every 4 weeks, starting at week 4.3
The Primary endpoint was to demonstrate the superiority of secukinumab over placebo, as assessed by the percentage change in total fingernail NAPSI score from baseline to week 16 =45.3, P<0.0001.3
*(HR 2.93, 95% CI 1.68 –5.12) than subjects without nail dystrophy.
†Mean baseline NAPSI score was 42.6.3
QW=every week; Q4W=every 4 weeks.
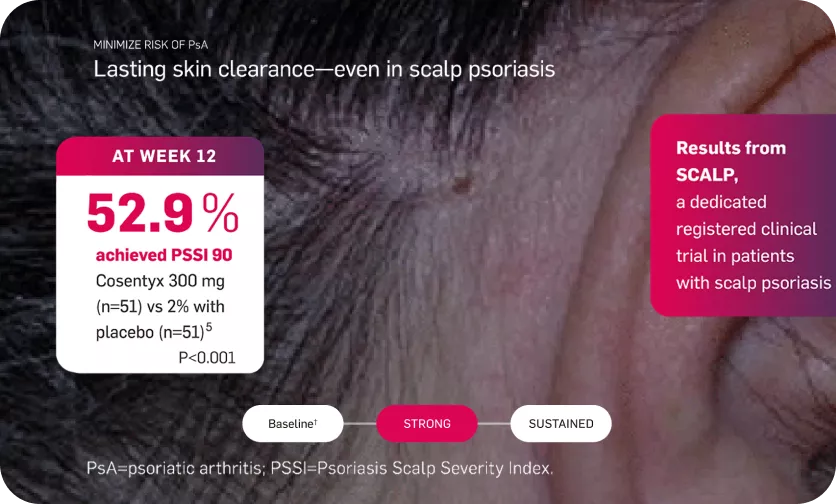
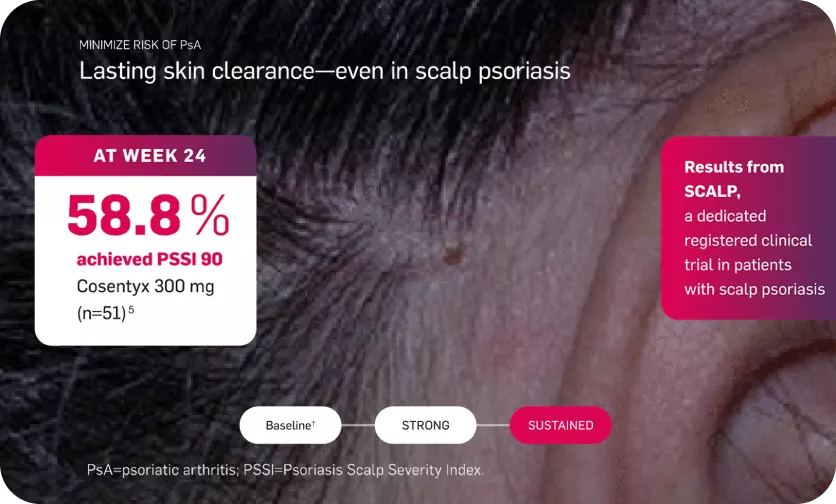
Lasting skin clearance—even in scalp psoriasis
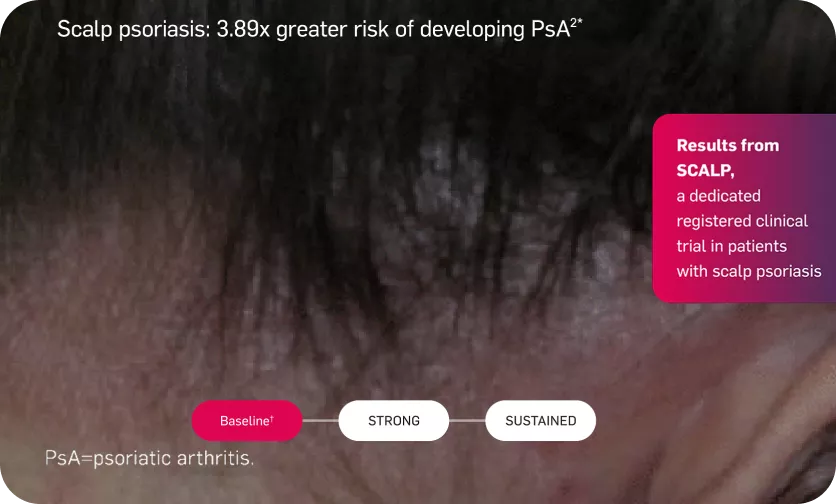
Adapted from reference 6
This 24-week randomized, double-blind, placebo-controlled, parallel-group, multicenter, phase 3b study was conducted according to the ethical principles of the Declaration of Helsinki and Good Clinical Practice.
Patients received Cosentyx 300 mg or placebo at baseline, weeks 1, 2, and 3, and then every 4 weeks from week 4 to 20. The final efficacy and safety evaluations were performed at week 24.5
Placebo-treated patients who achieved a PSSI improvement of 90% (PSSI 90) response at week 12 continued receiving placebo, and those who did not achieve a PSSI 90 response at week 12 switched to treatment with Cosentyx 300 mg at weeks 12, 13, 14, 15, 16, and 20.5
The primary objective was to determine the proportion of patients using Cosentyx 300 mg or patients using placebo who achieve a PSSI 90 score at week 12.
At week 12, PSSI 90 was achieved by 52.9% of patients receiving secukinumab 300 mg and by 2.0% of patients receiving placebo (difference between proportions: 0.51, 95% CI 0.37-0.65, P <.001)5
*(HR 3.89, 95% CI 2.18 – 6.94) compared with those without scalp lesions
†Mean baseline PSSI score was 33.5
PsA=psoriatic arthritis; PSSI=Psoriasis Scalp Severity Index; NRI=nonresponder imputation; QW=every week; Q4W=every 4 weeks.
Fast and lasting skin clearance
PASI 100 skin clearance rates over time
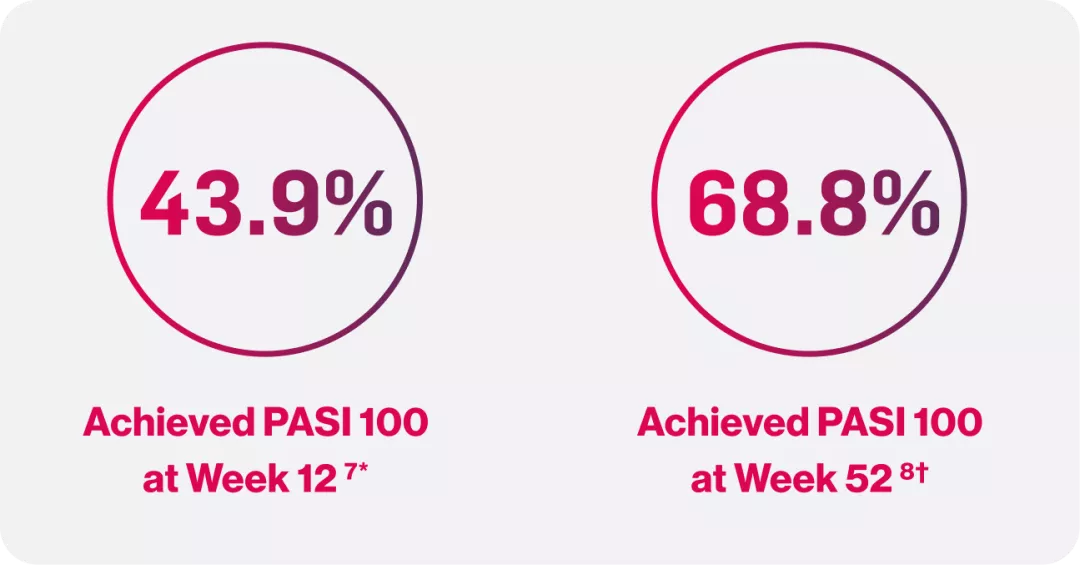
*At Week 12, total skin clearance (PASI100) was greater with Cosentyx treatment (300 mg 2 ml AI, 43.9% (p < 0.0001); 300 mg 2x1mLPFS,37.5%) than placebo(0%)7
†Early intervention delivers lasting results, with Cosentyx 300 mg Q4W led to a significantly higher PASI 100 response rate at Week 52 vs standard of care (nb- UVB phototherapy) (68.8% vs 22%; P<0.0001)8
PASI=Psoriasis Area and Severity Index; PsO=plaque psoriasis.

MATURE was a 52-week, multicenter, randomized, double-blind, placebo-controlled phase 3 trial that evaluated the efficacy, safety, tolerability, and PK of Cosentyx administered to patients with moderate to severe PsO via one 300-mg/2-mL Cosentyx® pen injection (n=41) or two 150-mg/1-mL PFS injections (n=41) vs placebo (n=40). Patients self-administered treatment at Weeks 0, 1,2, 3, 4, and 8, followed by Q4W dosing starting at Week 12 up to Week 48. The coprimary endpoints were PASI 75 and IGA mod 2011 0/1 response rates at Week 12. The key secondary endpoint was PASI 90 response rate at Week 12. Other secondary endpoints were evaluation of PK data and efficacy of Cosentyx at Week 52.8
SCULPTURE consisted of 2 parts: A 52-week core study (PASI 75 response rates in patients with moderate to severe PsO randomized to Cosentyx 150-mg or 300-mg fixed-interval dosing or to Cosentyx 150-mg or 300-mg retreatment-as-needed dosing) and a long-term extension study (N=168). Patients who completed 52 weeks of the core trial were eligible to enter the extension study. The trial was open label from Year 4. Long-term results were reported for the 300-mg fixed-interval, Q4W arm (labeled dose regimen) only.9
IGA mod 2011=Investigator’s Global Assessment modified 2011; PFS=prefilled syringe; PK=pharmacokinetics; PsO=plaque psoriasis;
Q4W=every 4 weeks.
Up to 41% of patients with PsO also have PsA10
Adapted from reference 12
If PsA left untreated, can lead to permanent joint damage and deformities.11
PsA=psoriatic arthritis; PsO=plaque psoriasis
PsA=psoriatic arthritis
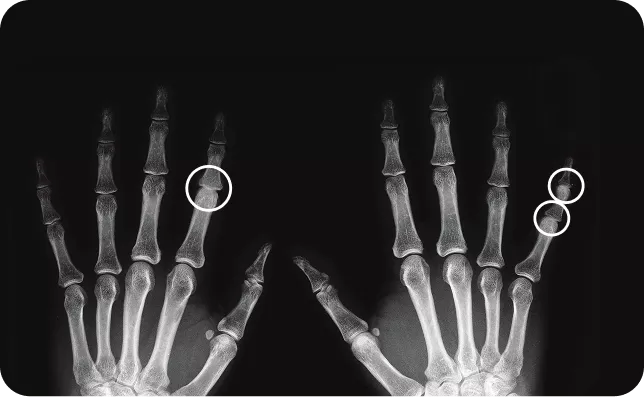
Cosentyx helps reduce the risk of irreversible joint damage
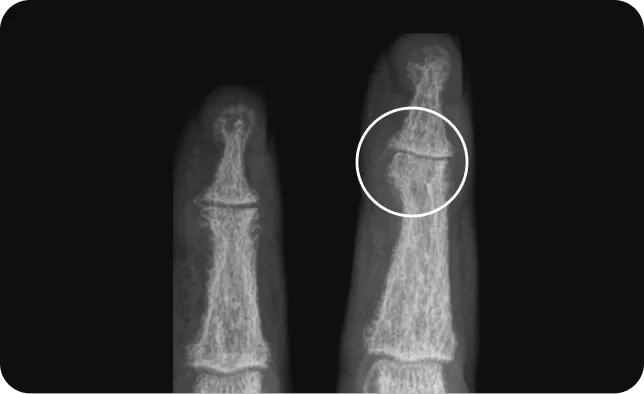
Adapted from reference 12
89.5% of patients with PsA showed no radiographic progression (change from baseline in vdH-mTSS 0.5) through week 10413
If PsA left untreated, can lead to permanent joint damage and deformities.11
Cosentyx API
Cosentyx API
References
Egyptian Drug Authority (EDA), Cosentyx leaflet approval date: 23/03/2025.
Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a populationbased study. Arthritis Care & Research. 2009 Feb 15;61(2):233-9.
Reich K, Sullivan J, Arenberger P, Mrowietz U, Jazayeri S, Augustin M, Parneix A, Regnault P, You R, Milutinovic M. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32week results from the randomized placebocontrolled TRANSFIGURE trial. British Journal of Dermatology. 2019 Nov 1;181(5):954-66.
Reich K, Sullivan J, Arenberger P, Jazayeri S, Mrowietz U, Augustin M, Elewski B, You R, Regnault P, Frueh JA. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5year results from the randomized placebocontrolled TRANSFIGURE study. British Journal ofDermatology. 2021 Mar 1;184(3):425-36.
Bagel J, Duffin KC, Moore A, Ferris LK, Siu K, Steadman J, Kianifard F, Nyirady J, Lebwohl M. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study.Journal of the American Academy of Dermatology. 2017 Oct 1;77(4):667-74.
American Academy of dermatology association. SCALP PSORIASIS: SHAMPOOS, SCALE SOFTENERS, AND OTHER TREATMENTS. Available at :https://www.aad.org/public/diseases/psoriasis/treatment/genitals/scalp-shampoo, Last Accessed: 26/09/2023.
Sigurgeirssn B, Browning J, Tyring S, Szepietowski JC, RiveraDíaz R, Effendy I, Keefe D, Bruin G, Paguet B, Fu R, Hampele I. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52week results from MATURE, a randomized, placebocontrolled trial. Dermatologic Therapy. 2022 Mar;35(3):e15285.
Iversen L, Conrad C, Eidsmo L, Costanzo A, Narbutt J, Pinter A, Kingo K, Rivera Diaz R, Kolbinger F, Nanna M, Frueh JA. Secukinumab demonstrates superiority over narrowband ultraviolet B phototherapy in newonset moderate to severe plaque psoriasis patients: Week 52 results from the STEPIn study. Journal of the European Academy of Dermatology and Venereology. 2023 May;37(5):1004-16.
Bissonnette R, Luger T, Thaçi D, Toth D, Lacombe A, Xia S, Mazur R, Patekar M, Charef P, Milutinovic M, Leonardi C. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderatetosevere psoriasis through 5 years of treatment (SCULPTURE Extension Study). Journal of the European Academy of Dermatology and Venereology. 2018 Sep;32(9):1507-14.
Gottlieb AB, Mease PJ, Mark Jackson J, Eisen D, Amy Xia H, Asare C, Stevens SR. Clinical characteristics of psoriatic arthritis and psoriasis in dermatologists' offices. Journal of dermatological treatment. 2006 Jan 1;17(5):279-87.
CreakyJoints.The Stages of Psoriatic Arthritis: Signs of Early to Late Disease Progression. Available at : https://creakyjoints.org/about-arthritis/psoriatic-arthritis/psa-overview/psoriatic-arthritis-stages-progression, last Accessed:18/06/2025.
Shiraishi M, Fukuda T, Igarashi T, Tokashiki T, Kayama R, Ojiri H. Differentiating rheumatoid and psoriatic arthritis of the hand:multimodality imaging characteristics. Radiographics. 2020 Sep;40(5):1339-54.
Mease PJ, Landewé R, Rahman P, Tahir H, Singhal A, Boettcher E, Navarra S, Readie A, Mpofu S, Delicha EM, Pricop L. Secukinumab provides sustained improvement in signs and symptoms and low radiographic progression in patients with psoriatic arthritis: 2-year(end-of-study) results from the FUTURE 5 study. RMD open. 2021 Jul 1;7(2):e001600.
Approved by Egyptian Drug Authority: BF0424OA4706/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
BF0424OA4706/082025 28/08/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
