
For adult patients with moderate to severe plaque psoriasis (PsO) who are candidates for systemic therapy1
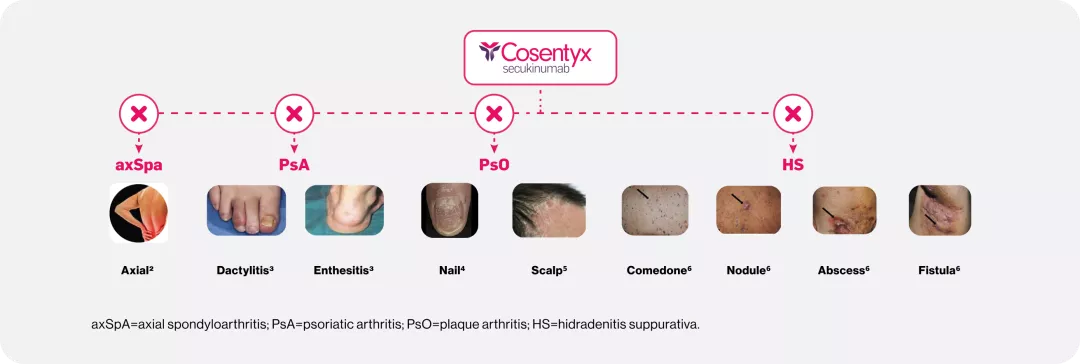
Cosentyx blocks IL-17A, a key cytokine responsible for inflammation that underlies axSpA, PsA, PsO, and HS1
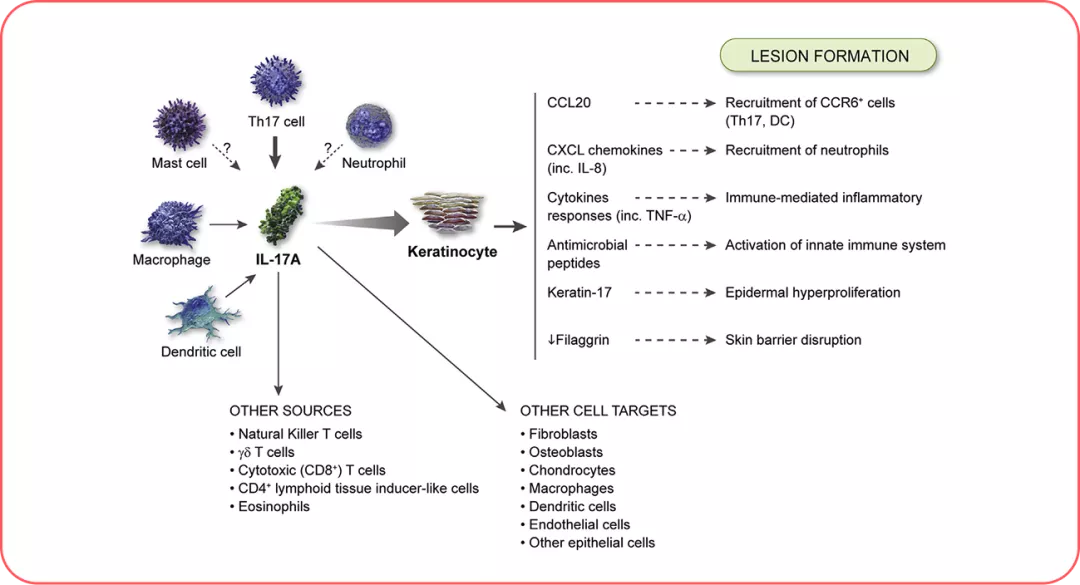
Adapted from Ref.7
Cellular sources and targets in psoriasis. T-helper (Th)17 cells are the key cellular sources, and keratinocytes are the key cellular target of interleukin (IL)-17A. Neutrophils stain positive for IL-17, but production of the protein has yet to be confirmed. Similarly, some evidence suggests that mast cells produce IL-17A, but further investigation is required. 7
IL=interleukin; axSpA=axial spondyloarthritis; PsA=psoriatic arthritis; PsO=plaque arthritis; HS=hidradenitis suppurativa.
Increased levels of IL-17A are found in the affected tissues of patients with axSpA, PsA, PsO, HS1
Image

| Across 8 indications1 |








The complete Cosentyx approach
Cosentyx API
Cosentyx API
References
Egyptian Drug Authority (EDA), Cosentyx leaflet approval date: 23/03/2025.
What Is Axial Spondyloarthritis_ Symptoms, Causes, Diagnosis. Creakyjoints organization. Available at:https://creakyjoints.org/about-arthritis/axial-spondyloarthritis/axspa-overview/what-is-axial-spondyloarthritis/; last accessed :19-06-2025.
Bagel J, Schwartzman S. Enthesitis and dactylitis in psoriatic disease: a guide for dermatologists. American Journal of clinical dermatology. 2018 Dec;19(6):839-52.
What is nail psoriasis, and how can I treat it. American Academy of Dermatology. Available at: https://www.aad.org/public/diseases/psoriasis/treatment/genitals/nails; last accessed: 19-06-2025.
Scalp Psoriasis. Symptoms, Plaque, Causes & Treatment. Available at: https://my.clevelandclinic.org/health/diseases/22828-scalp-psoriasis; last accessed: 19-06-2025.
Hidradenitis suppurativa – Management, comorbidities and monitoring. RACGP. Available at: https://www.racgp.org.au/afp/2017/august/hidradenitis-suppurativa-management-comorbidities; Last accessed 19-06-2025.
Iversen L, Conrad C, Eidsmo L, Costanzo A, Narbutt J, Pinter A, Kingo K, Rivera Diaz R, Kolbinger F, Nanna M, Frueh JA. Secukinumab demonstrates superiority over narrowband ultraviolet B phototherapy in newonset moderate to severe plaque psoriasis patients: Week 52 results from the STEPIn study. Journal of the European Academy of Dermatology and Venereology. 2023 May;37(5):1004-16.
Gottlieb AB, Deodhar A, Mcinnes IB, Baraliakos X, Reich K, Schreiber S, Bao W, Marfo K, Richards HB, Pricop L, Shete A. Long-term safety of secukinumab over five years in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis and ankylosing spondylitis: update on integrated pooled clinical trial and post-marketing surveillance data. Acta Dermato Venereologica. 2022 Apr 27;102.
Approved by Egyptian Drug Authority: BF0424OA4706/082025. Invalidation date: 28/08/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
BF0424OA4706/082025 28/08/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |



