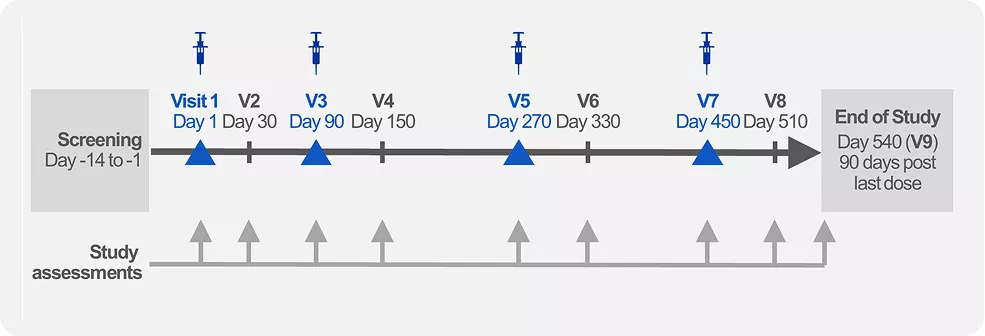
Orion 10
Results: Efficacy1
After 17 months, between-groups treatment difference in LDL-C was −52.3%
Change in LDL-C over time – observed values in ITT population*
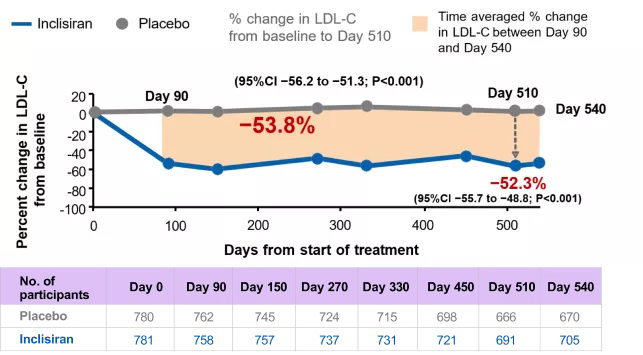
*Using observed numbers and not imputed for missing data
After 17 months, inclisiran achieved 83.3% reduction in PCSK9 levels
Change in PCSK9 over time – observed values in ITT population*
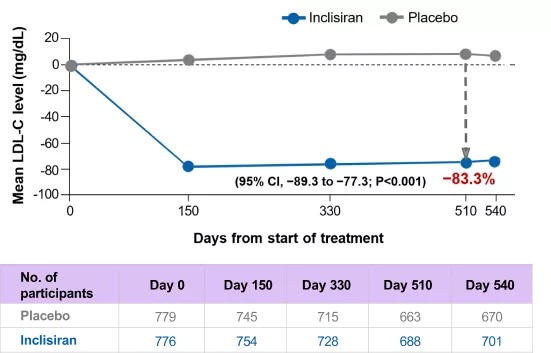
*Using observed numbers and not imputed for missing data
Orion 11
Results: Efficacy1
After 18 months, between-groups treatment difference in LDL-C was −50%
Change in LDL-C over time – observed values in ITT population*
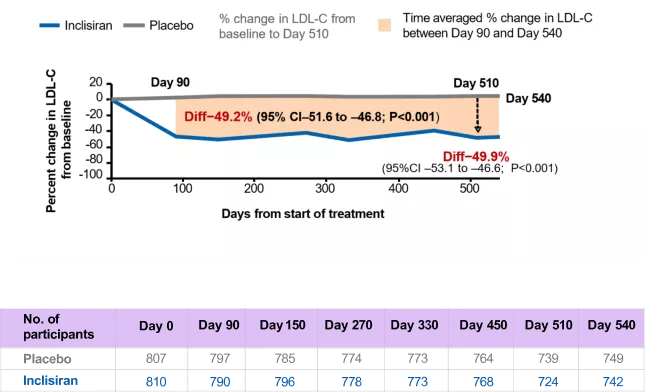
*Using observed numbers and not imputed for missing data
After 18 months, inclisiran achieved 79.3% change reduction in PCSK9 levels
Change in PCSK9 over time – observed values in ITT population*
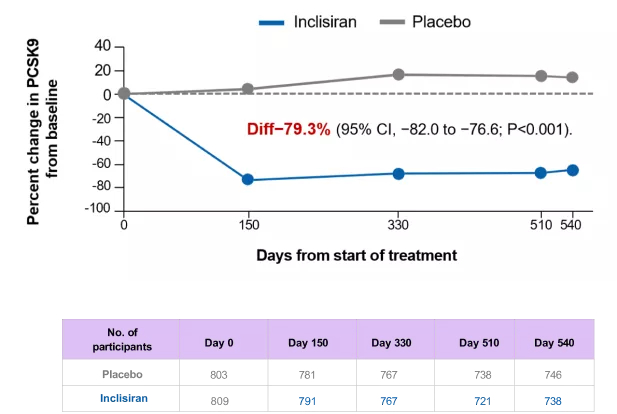
*Using observed numbers and not imputed for missing data
Orion 8
Results: Efficacy2
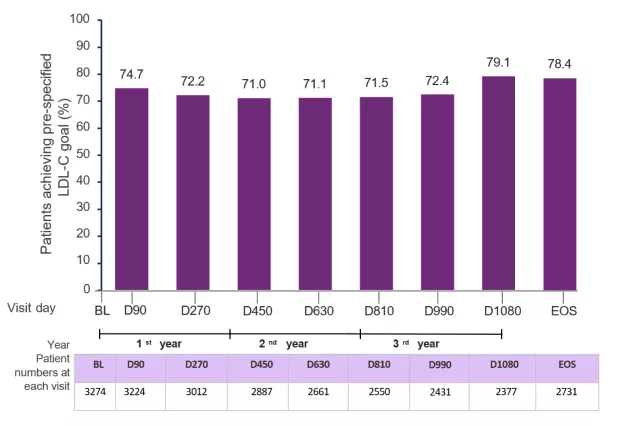
The proportion (95% CI) of patients achieving the primary pre-specified LDL-C goal at end-of-study was 78.4% (95% CI: 76.8–80.0) of 2731 patients.
At each visit in ORION-8, more than 70% of the patients achieved the prespecified LDL-C goals.
Overall safetypopulation
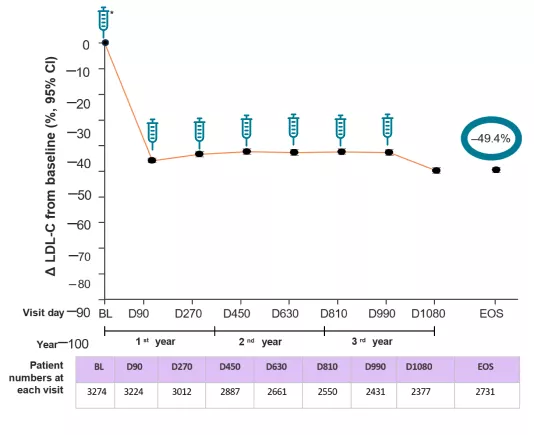
*Baseline value of LDL-C was taken from the baseline of the parent trials.
The percentage change from the parent trial baseline in LDL-C over time
Overall safetypopulation
Orion 9
Results: Efficacy3
For the first primary end point, the percent change in the LDL cholesterol level from baseline to day 510 was a decrease of 39.7% (95% confidence interval [CI], −43.7 to −35.7) in the inclisiran group and an increase of 8.2% (95% CI, 4.3 to 12.2) in the placebo group, for a between-group difference of −47.9 percentage points (95% CI, −53.5 to −42.3; P<0.001)
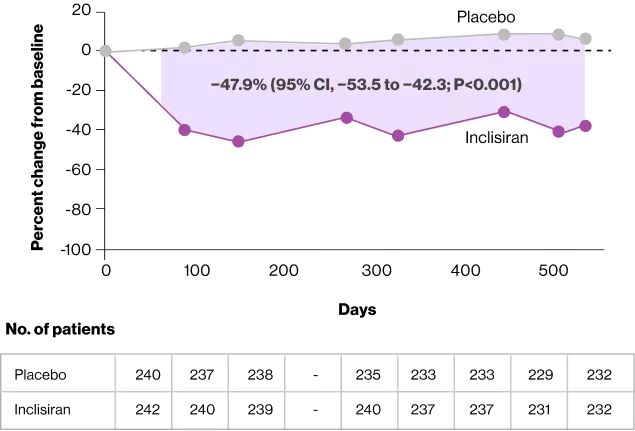
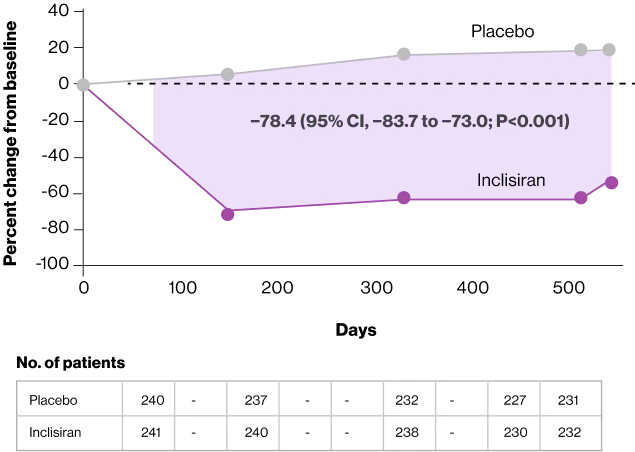
At day 510, the percent change in the PCSK9 level was a decrease of 60.7% (95% CI, −64.4 to −57.0) in the inclisiran group and an increase of 17.7% (95% CI, 13.9 to 21.4) in the placebo group for a between-group difference of −78.4 percentage points (95% CI, −83.7 to −73.0; P<0.001)
Leqvio® API
Leqvio® API
References
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. New England journal of medicine. 2020 Apr 16;382(16):1507-19.
Wright RS, Raal FJ, Koenig W, et al. Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: the ORION-8 trial. Cardiovascular research. 2024 Aug;120(12):1400-10
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. New England Journal of Medicine. 2020 Apr 16;382(16):1520-30.
Ray KK, Wright RS, Kallend D, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol (supplementary appendix). New England journal of medicine. 2020 Apr 16;382(16):1507-19.
Raal FJ, Kallend D, Ray KK, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia (supplementary appendix). New England Journal of Medicine. 2020 Apr 16;382(16):1520-30.
Approved by Egyptian Drug Authority: HF0424OA4729/092025. Invalidation date: 23/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0424OA4729/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |