LEQVIO®▼ (inclisiran)
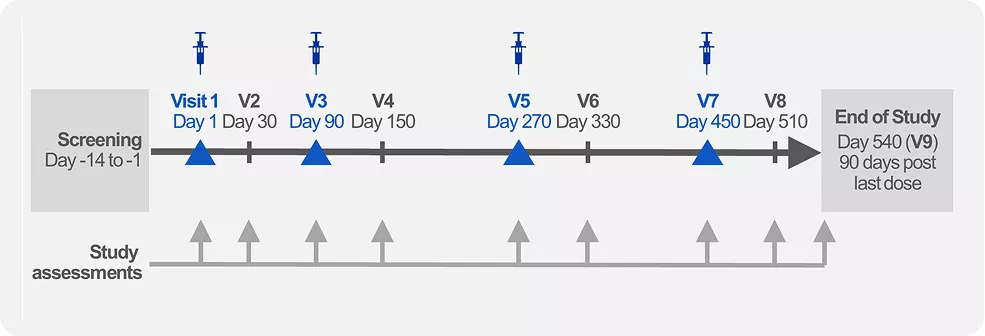
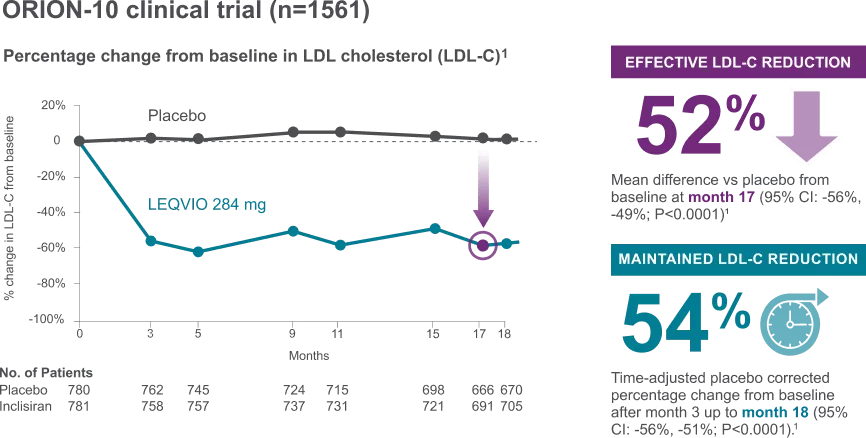
Leqvio gives you the assurance of effective and long-lasting LDL-c reduction as effectiveness maintained during long term therapy.1
Administration of 2 maintenance doses per year by a healthcare professional gives control over adherence. 1,4
LEQVIO PROVIDED SIGNIFICANT LDL-C REDUCTION FOR PATIENTS TO REACH AND STAY ON TARGET¹
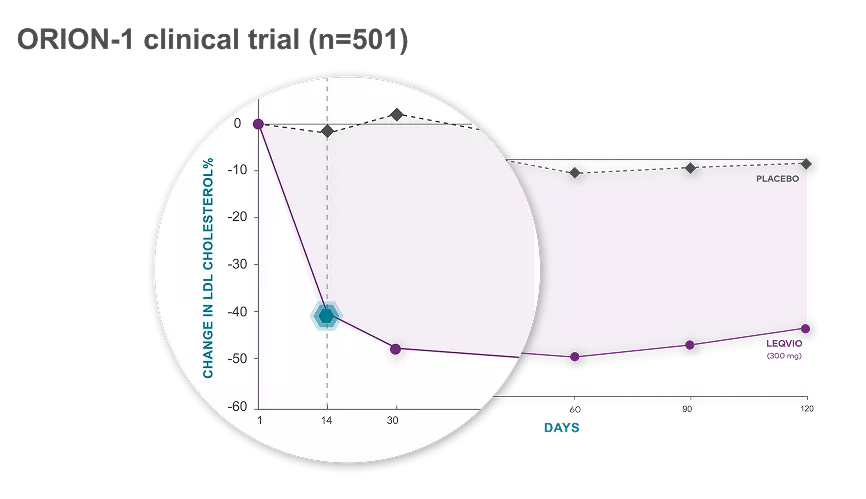
Rapid Onset
LDL-C levels dropped significantly 14 days after the first injection.2
Reductions range from 44.5% to 50.5% below baseline by day 30 across all doses.2
PATIENTS ON LEQVIO DEMONSTRATED LDL-C REDUCTION AT TWO WEEKS2
SAFETY
Well-tolerated3
The safety of Leqvio was evaluated in 3 Phase III placebo-controlled trials that included 3,655 patients with atherosclerotic cardiovascular disease (ASCVD), ASCVD risk equivalents, or familial hypercholesterolemia, treated with maximally tolerated statins and Leqvio or placebo, including 1,833 patients exposed to inclisiran for up to 18 months (mean treatment duration of 526 days).1
Safety data from the 3 Phase Ill placebo-controlled pivotal trials showed that treatment-emergent adverse events (TEAEs) occurred at a similar incidence in the Leqvio-treated and placebo-treated patients.1
The majority of the TEAEs were mild and unrelated to Leqvio or placebo.1
The only adverse reactions associated with Leqvio in pivotal trials were adverse events at the injection site.1
Leqvio® API
Leqvio® API
References
Egyptian Drug Authority (EDA). Leqvio leaflet approval date: 2/4/2024.
Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. New England Journal of Medicine. 2017;376(15):1430-1440.
Wright RS, Ray KK, Raal FJ, et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. Journal of the American College of Cardiology. 2021 Mar 9;77(9):1182-93.
Leqvio, Summary of product charactristics (SMPC). available at: https://www.ema.europa.eu/en/documents/product-information/leqvio-epar-product-information_en.pdf. last accessed:
11/6/2025
Approved by Egyptian Drug Authority: HF0424OA4729/092025. Invalidation date: 23/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg
Image

|
HF0424OA4729/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |