
Unmet need in CML treatment post 2 TKIs
Many patients switch treatment due to resistance or intolerance, but using treatment after treatment of TKIs may lead to low response rates and decreased survival1-3
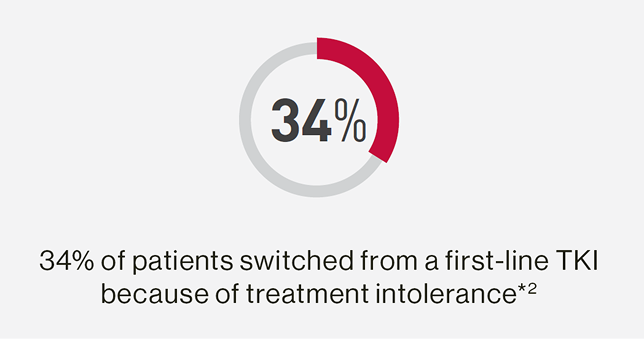
In a real-world study in the UK:


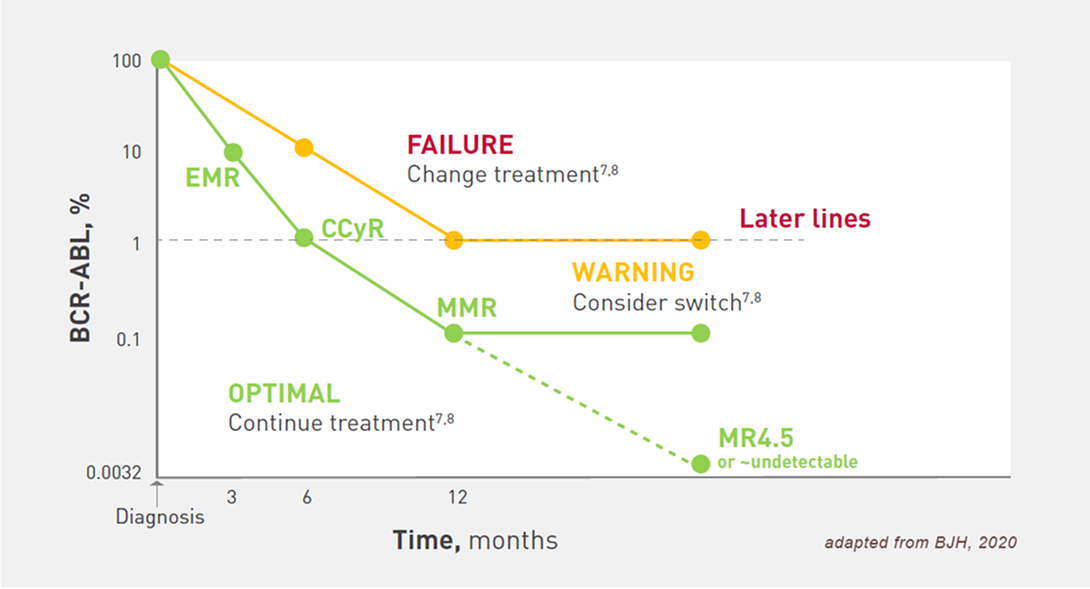
Regular monitoring is important to assess treatment benefits and inform the decision to switch7,8
Monitoring milestones of BCR-ABL1 transcript levels by the IS at 3,6, and 12 months is essential to determine treatment interventions7. In later treatments, acceptable response cannot be formally defined, but a BCR-ABL1 >1% is insufficient for optimal survival.7,8
SCEMBLIX is the first and only STAMP inhibitor, with a unique MOA4
SCEMBLIX showed superior efficacy vs bosutinib as early as week 245
AE, adverse event; CML, chronic myeloid leukaemia; MOA, mechanism of action; MMR, major molecular remission; Ph+ CML-CP, Philadelphia chromosome-positive chronic myeloid leukaemia in chronic phase; STAMP, Specifically Targeting the ABL1 Myristoyl Pocket; TKI, tyrosine kinase inhibitor; URTI, upper respiratory tract infection.
*Data from a retrospective, non-interventional study conducted at 21 UK NHS secondary and tertiary care centres on 257 CML patients.2
SCEMBLIX is indicated for the treatment of patients 18 years of age and above with:
- Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP).
- Previously treated Ph+ CML in CP.
- Ph+ CML in CP with the T315I mutation
SCEMBLIX™ 20mg and 40mg NSS
SCEMBLIX™ 20mg and 40mg NSS
References
Bosi GR, Fogliatto LM, Costa TEV, et al. Hematol Transfus Cell Ther. 2019;41(3):222 228.
Milojkovic D, Cross NCP, Ali S, Byrne J, et al. Br J Haematol, 2021;192:62–74.
Garg RJ, Kantarjian H, O’Brien S, et al. Blood. 2009;114(20):4361-4368.
Hughes TP, Mauro MJ, Cortes JE, et al. N Engl J Med. 2019;381(24):2315-2326.
Réa D, Mauro MJ, Boquimpani C, et al. Blood. 2021 Nov 25;138(21):2031-2041.
SCEMBLIX (asciminib) Approved Product Information.
Hochhaus A et al. Leukaemia 2020;34:966–84.
Smith G et al. Br J Haematol 2020;191(2):171–93.
