
The ASCEMBL trial: safety and tolerability profile
Patients receiving SCEMBLIX and bosutinib, respectively, experienced:2
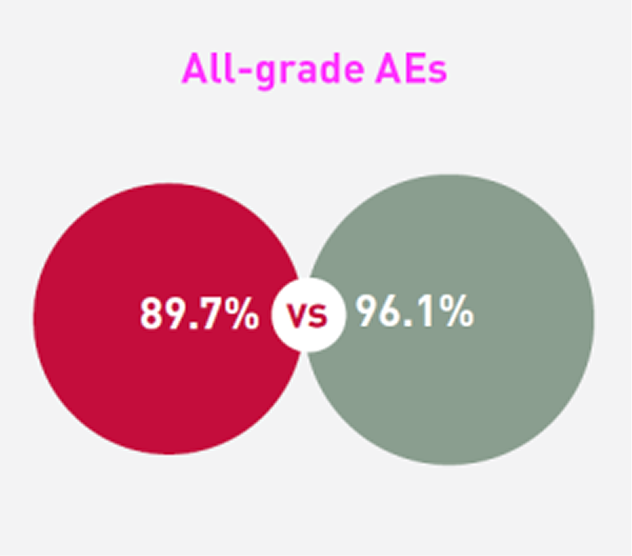
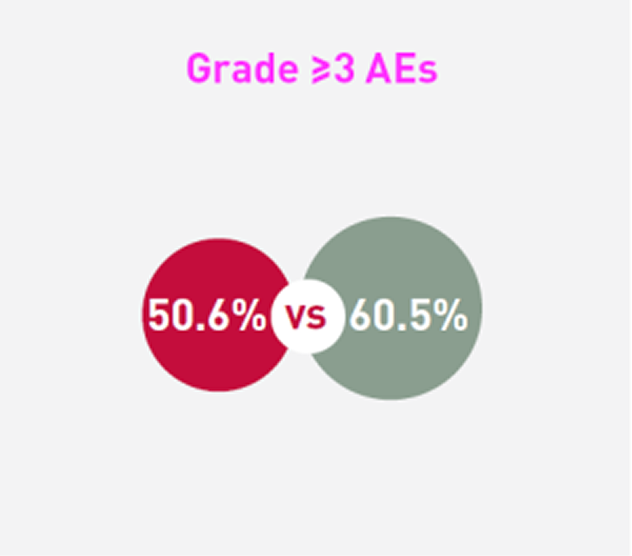
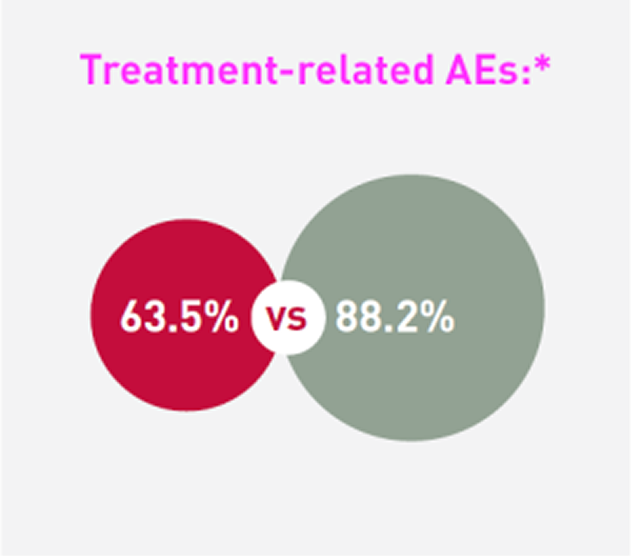
*The cut-off date for this analysis was 25 May 2020. The study is ongoing. The median duration of follow-up was 14.9 months from randomisation to data cut-off date.
Most frequent all-grade AEs (occurring in ≥10% of patients in any treatment arm)1
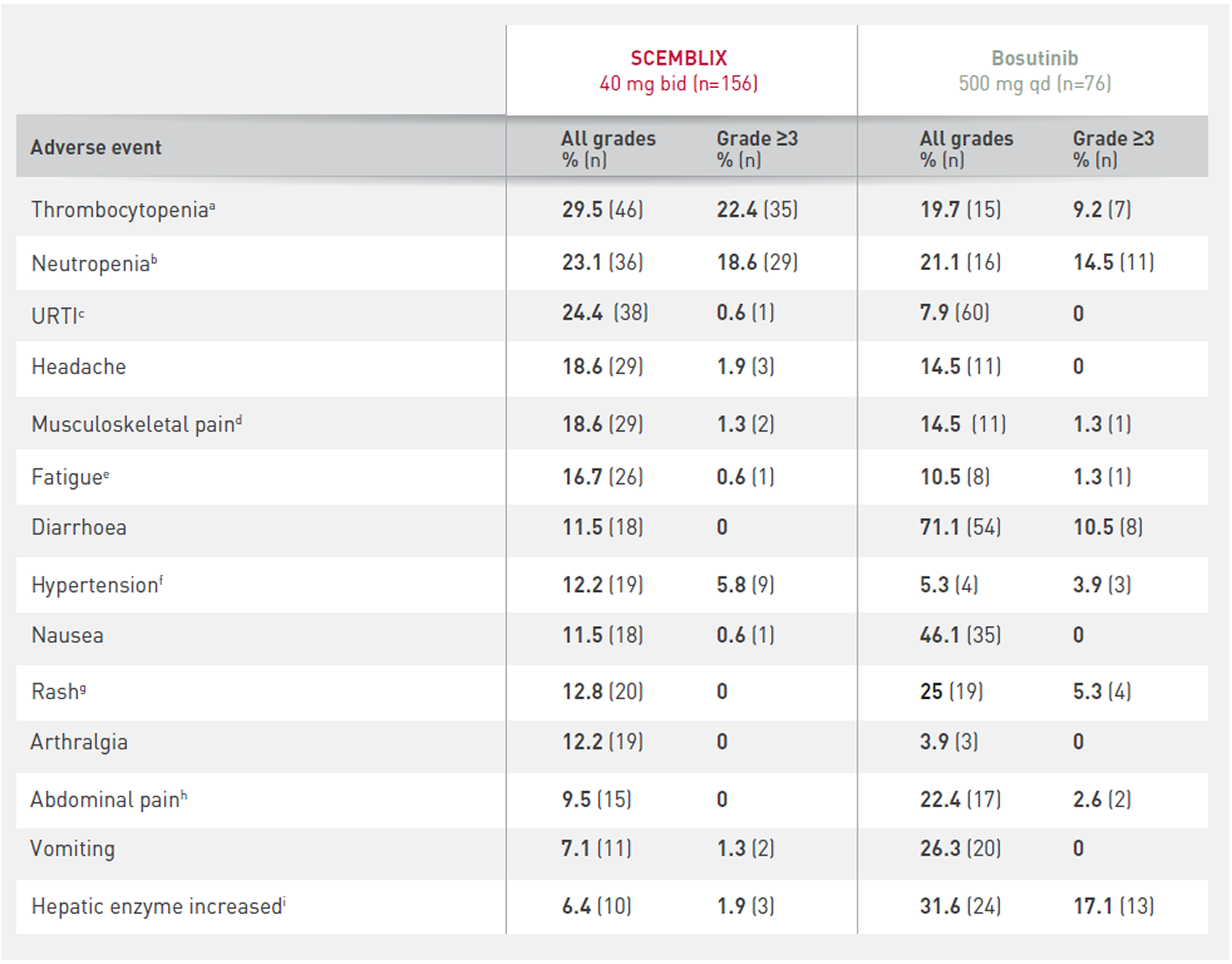
* Per investigator assessment.
a Thrombocytopenia includes: thrombocytopenia and platelet count decreased.
b Neutropenia includes: neutropenia and neutrophil count decreased.
c URTI includes: upper respiratory tract infection, nasopharyngitis, pharyngitis and rhinitis.
d Musculoskeletal pain includes: pain in extremity, back pain, myalgia, bone pain, musculoskeletal pain, neck pain, musculoskeletal chest pain and musculoskeletal discomfort.
e Fatigue includes: fatigue and asthenia.
f Hypertension includes: hypertension and blood pressure increased.
g Rash includes: rash and rash maculopapular.
h Abdominal pain includes: abdominal pain and abdominal pain upper.
i Hepatic enzymes increased includes: alanine aminotransferase increased, aspartate aminotranferase increased, gamma-glutamyltransferase increased and transaminases increased.

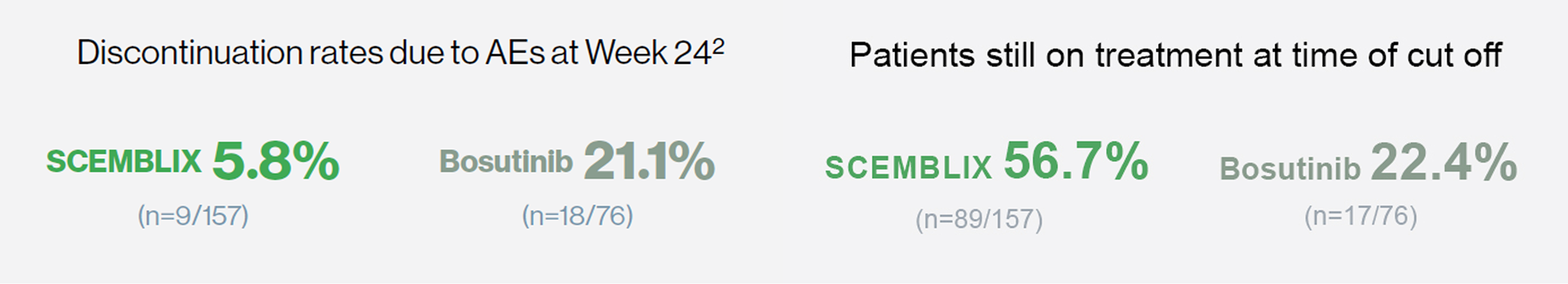
Patients taking SCEMBLIX experienced nearly four times lower treatment discontinuation vs bosutinib1

Switch your post 2 TKI CML patients with confidence
AE, adverse event; CML, chronic myeloid leukaemia; MOA, mechanism of action; MMR, major molecular remission; Ph+ CML-CP, Philadelphia chromosome-positive chronic myeloid leukaemia in chronic phase; STAMP, Specifically Targeting the ABL1 Myristoyl Pocket; TKI, tyrosine kinase inhibitor; URTI, upper respiratory tract infection. CCyR, complete cytogenetic remission; CML-CP, chronic myeloid leukaemia in chronic phase; IS, international scale; MCyR, major cytogenetic response; MMR, major molecular response; STAMP, specifically targeting the ABL1 myristoyl pocket; TKI, tyrosine kinase.
SCEMBLIX is indicated for the treatment of patients 18 years of age and above with:
- Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP).
- Previously treated Ph+ CML in CP.
- Ph+ CML in CP with the T315I mutation
SCEMBLIX™ 20mg and 40mg NSS
SCEMBLIX™ 20mg and 40mg NSS
References
SCEMBLIX (asciminib) approved Product Information.
Réa D, Mauro MJ, Boquimpani C, et al. Blood. 2021;138(21):2031-2041.
Mauro MJ, Minami Y, Réa D et al. Presented at: American Society for Hematology 63rd Annual Meeting; December 11-14, 2021 [Oral presentation 310].
