
*EARLY,† DURABLE‡ and DEEPER# responses with a favourable tolerability profile vs bosutinib, a 2nd-generation TKI2,4
†MMR difference as early as Week 12.4 ‡Increased MMR benefit at week 24 maintained at week 96.2,4 #Increased MR4 and MR4.5 at Week 24 and Week 96.2,4.
How SCEMBLIX (asciminib) works
The first and only approved BCR-ABL1 inhibitor specifically targeting the ABL1 myristoyl pocket (STAMP)2,3,5
SCEMBLIX targets a different site on the BCR-ABL1 compared to TKIs - the myrlstoyl pocket2,3,5
In people who do not have CML, the myristoyl pocket is occupied by the N-terminal portion of ABL1, maintaining the protein in an inactive conformation.6,7
In patients with CML, the myristoyl pocket on BCR-ABL1 is vacant, keeping the kinase in an active conformation.6,7
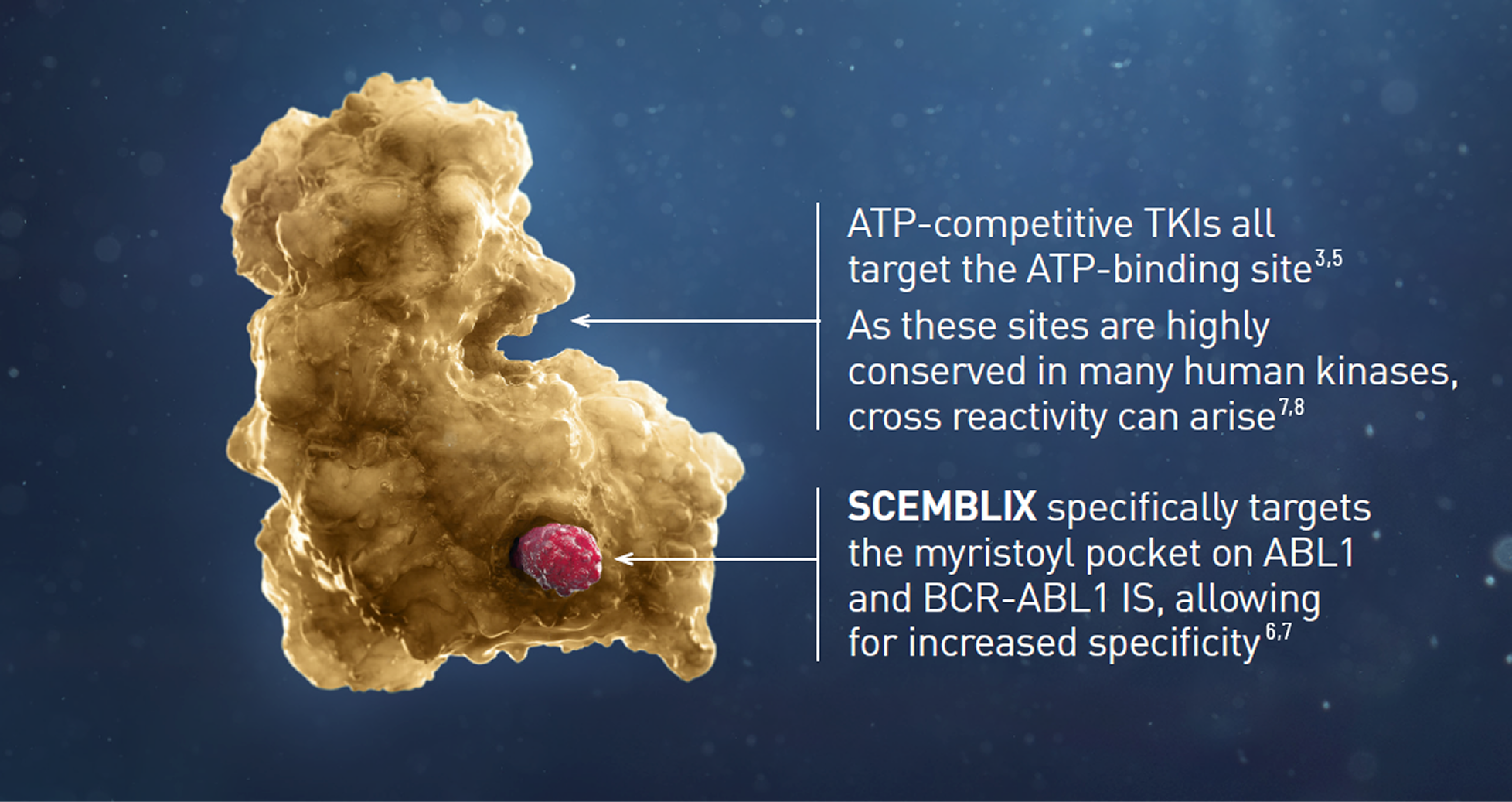
SCEMBLIX is a first-in-class STAMP inhibitor. Binding specifically to the myristoyl pocket, it potently inactivates BCR-ABL1 via allosteric inhibition.2

AE, adverse event; CML, chronic myeloid leukaemia; MOA, mechanism of action; MMR, major molecular remission; Ph+ CML-CP, Philadelphia chromosome-positive chronic myeloid leukaemia in chronic phase; STAMP, Specifically Targeting the ABL1 Myristoyl Pocket; TKI, tyrosine kinase inhibitor; URTI, upper respiratory tract infection.
*Data from a retrospective, non-interventional study conducted at 21 UK NHS secondary and tertiary care centres on 257 CML patients.2
SCEMBLIX is indicated for the treatment of patients 18 years of age and above with:
- Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP).
- Previously treated Ph+ CML in CP.
- Ph+ CML in CP with the T315I mutation
SCEMBLIX™ 20mg and 40mg NSS
SCEMBLIX™ 20mg and 40mg NSS
References
SCEMBLIX (asciminib) approved Product Information.
Réa D. Mauro M.J. Boquimpani C, et al. Blood 2021;138(21):2031–41.
Mauro M.J.Minami Y, Réa D. et al. Presented at: American Society for Hematology 63rd Annual Meeting ; December 11-14,2021 [Oral presentation 310].
