
SCEMBLIX (asciminib) has simple, once or twice-daily oral dosing1
SCEMBLIX is indicated for the treatment of patients 18 years of age and above with:1
- Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic phase (CP) previously treated with two or more tyrosine kinase inhibitors.
- Ph+ CML in CP with the T315I mutation.
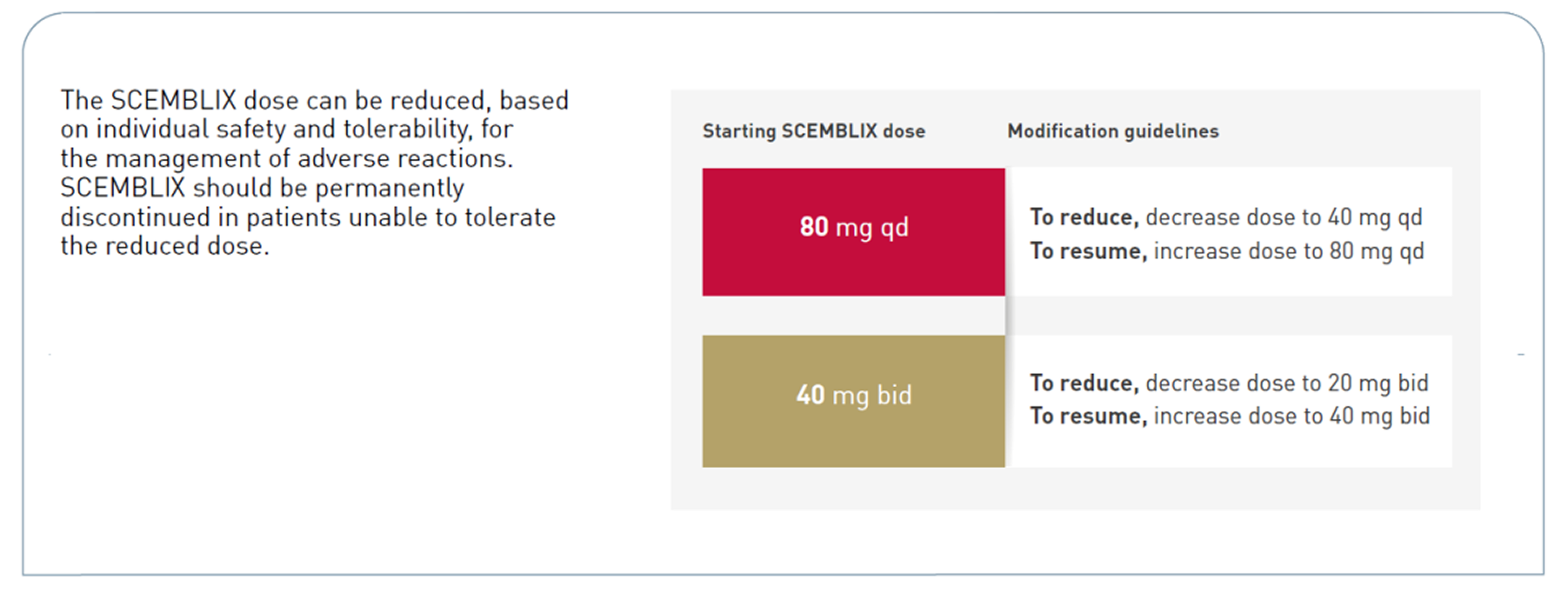
Recommended dosage in Ph+ CML-CP, previously treated with ≥2 TKIs1
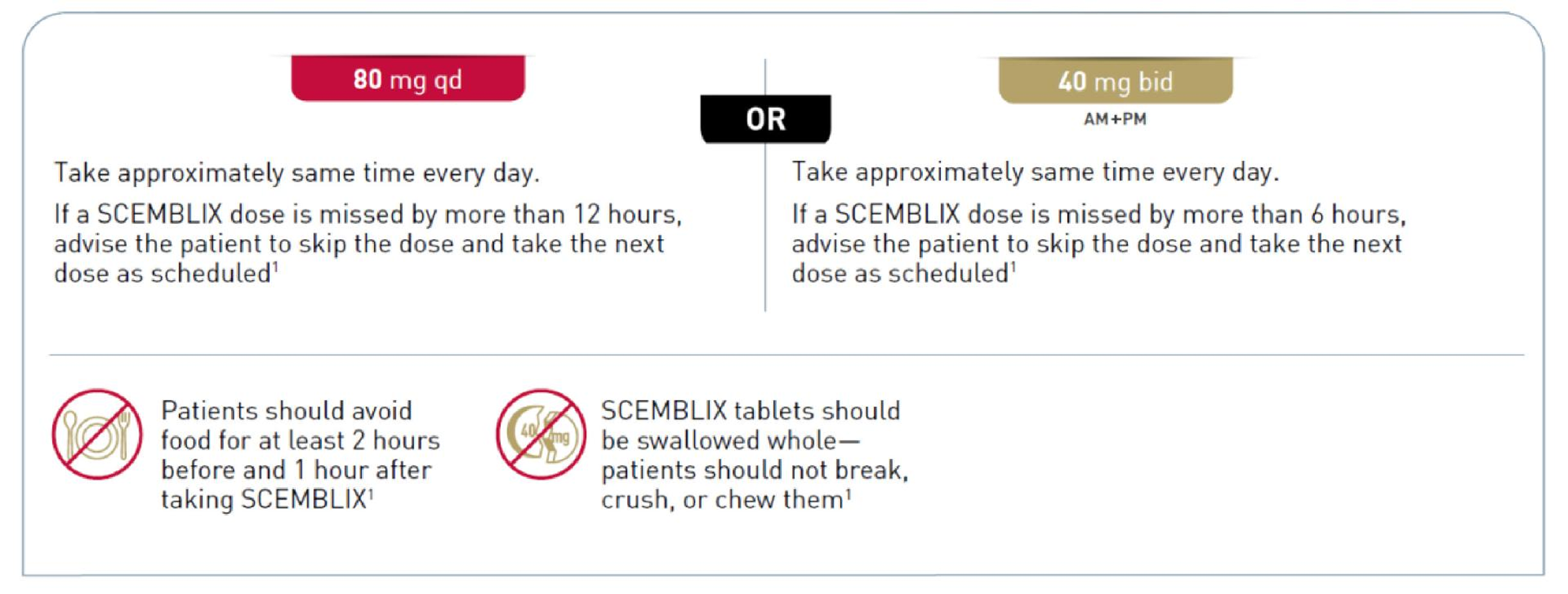
Ph+ CML CP harbouring the T315I mutation The recommended dose of SCEMBLIX is 200 mg taken orally twice daily at approximately 12 hour intervals.1
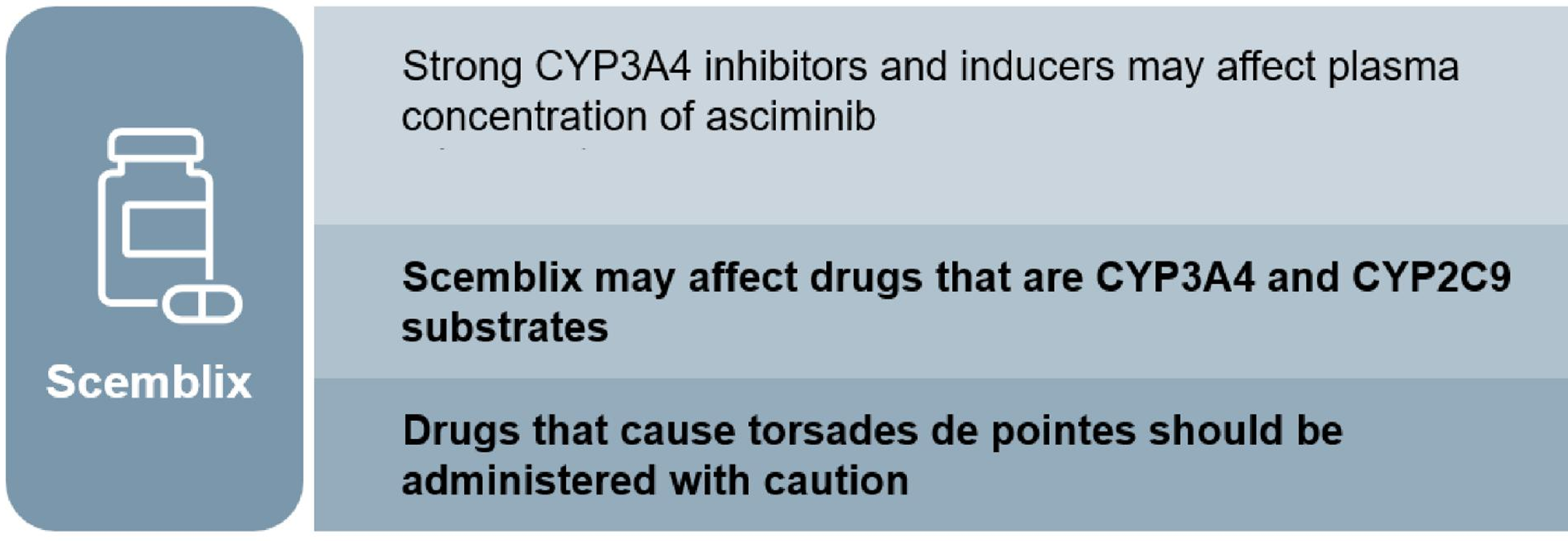

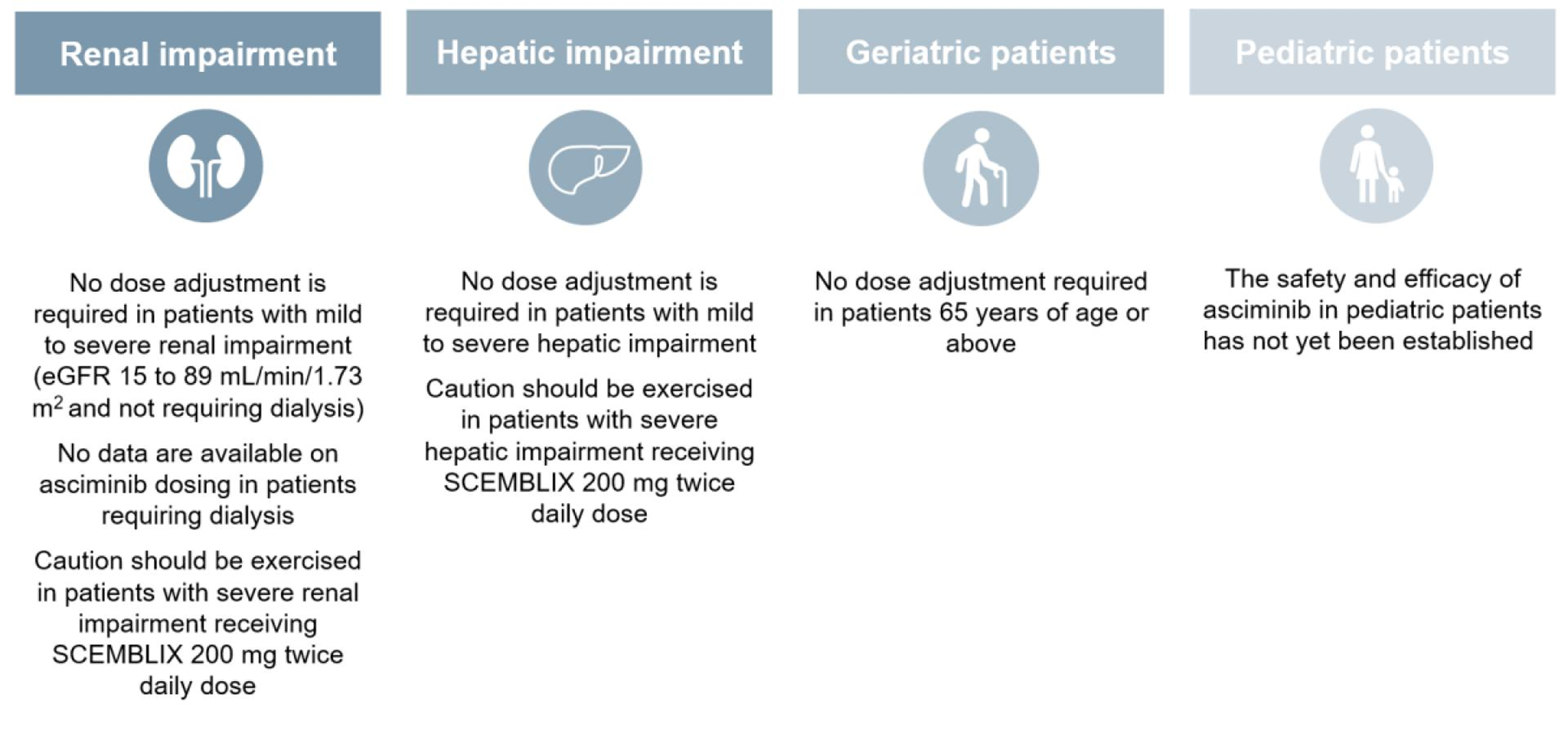
AE, adverse event; CML, chronic myeloid leukaemia; MOA, mechanism of action; MMR, major molecular remission; Ph+ CML-CP, Philadelphia chromosome-positive chronic myeloid leukaemia in chronic phase; STAMP, Specifically Targeting the ABL1 Myristoyl Pocket; TKI, tyrosine kinase inhibitor; URTI, upper respiratory tract infection. CCyR, complete cytogenetic remission; CML-CP, chronic myeloid leukaemia in chronic phase; IS, international scale; MCyR, major cytogenetic response; MMR, major molecular response; STAMP, specifically targeting the ABL1 myristoyl pocket; TKI, tyrosine kinase; BID, twice daily; QD, once daily; CYP, cytochrome P450; BCRP, breast cancer resistance protein; UGT, UDP glucuronosyltransferase.
SCEMBLIX is indicated for the treatment of patients 18 years of age and above with:1
- Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP).
- Previously treated Ph+ CML in CP.
- Ph+ CML in CP with the T315I mutation
SCEMBLIX™ 20mg and 40mg NSS
SCEMBLIX™ 20mg and 40mg NSS
References
SCEMBLIX (asciminib) approved Product Information.
