
The ASCEMBL trial: efficacy
ASCEMBL is the first head-to-head Phase 3 study comparing a CML treatment vs bosutinib, a 2nd-generation TKI1,3,9
A multicentre, randomised, active-controlled and open-label Phase 3 study of SCEMBLIX vs bosutinib, assessing MMR at 24 and 48 weeks , and other endpoints including MMR, CCyR and safety.1,10
SCEMBLIX nearly doubled the MMR rate vs bosutinib at Week 24 (primary endpoint)1
Achieving MMR has been associated with superior long-term outcomes, including survival and progression-free survival.1
MMR at Week 24 [primary endpoint]1
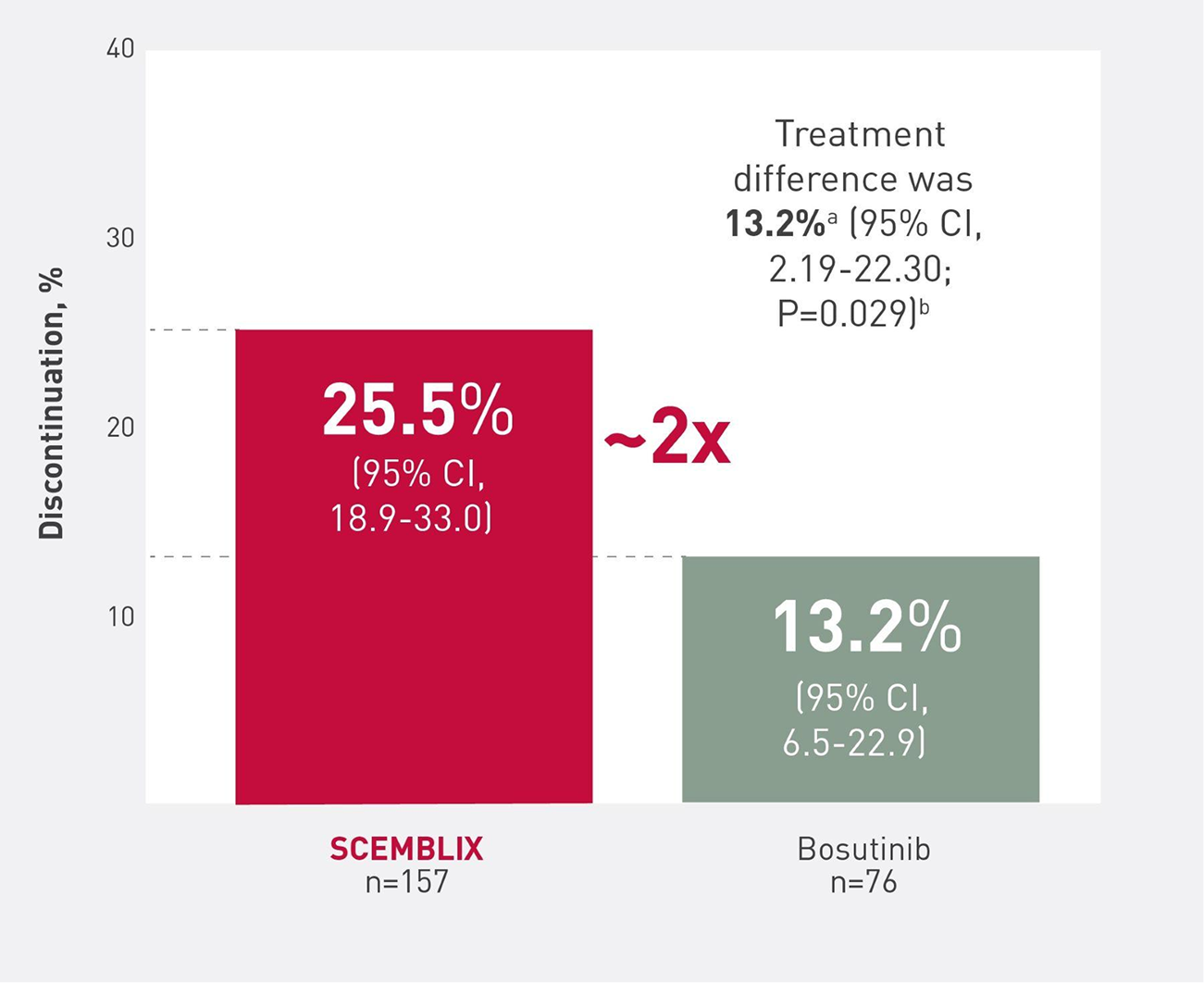

MMR Continues to be higher with SCEMBLIX vs bosutinib at week 4810
MMR at week 483
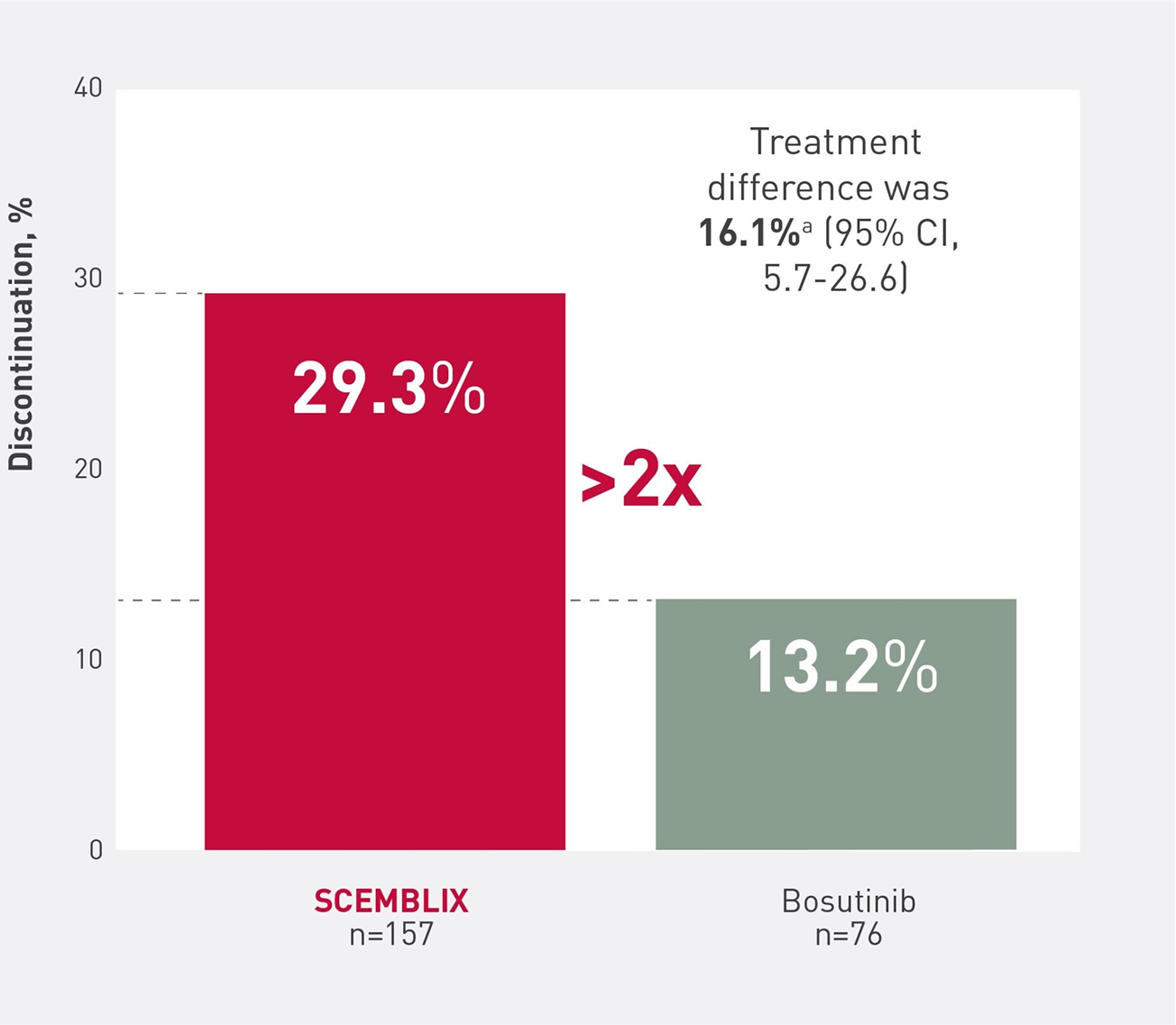

SCEMBLIX consistently increased cumulative MMR vs bosutinib over time1,2
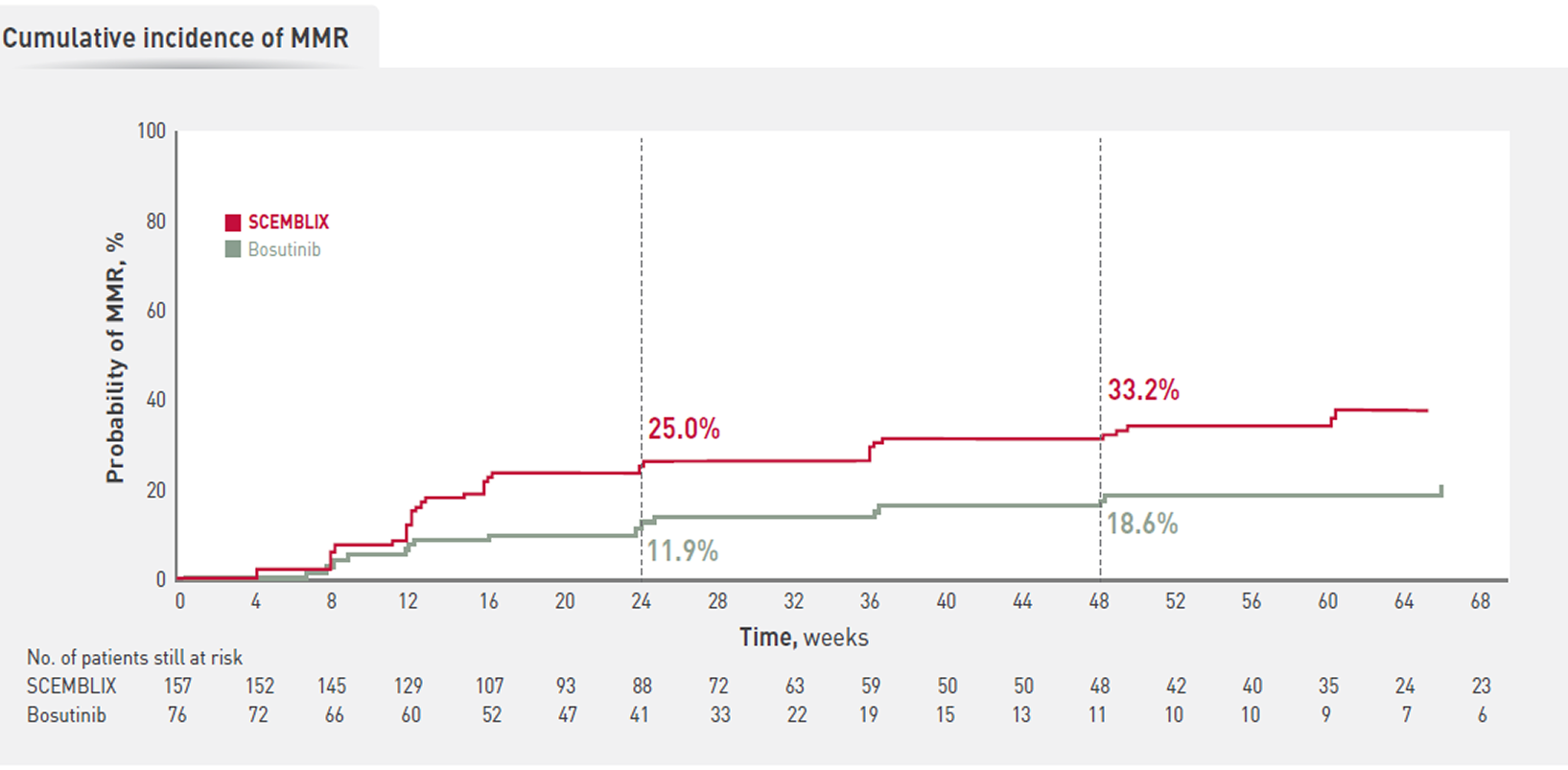

With SCEMBLIX, more patients are likely to achieve deep molecular response vs bosutinib at weeks 24 and 481
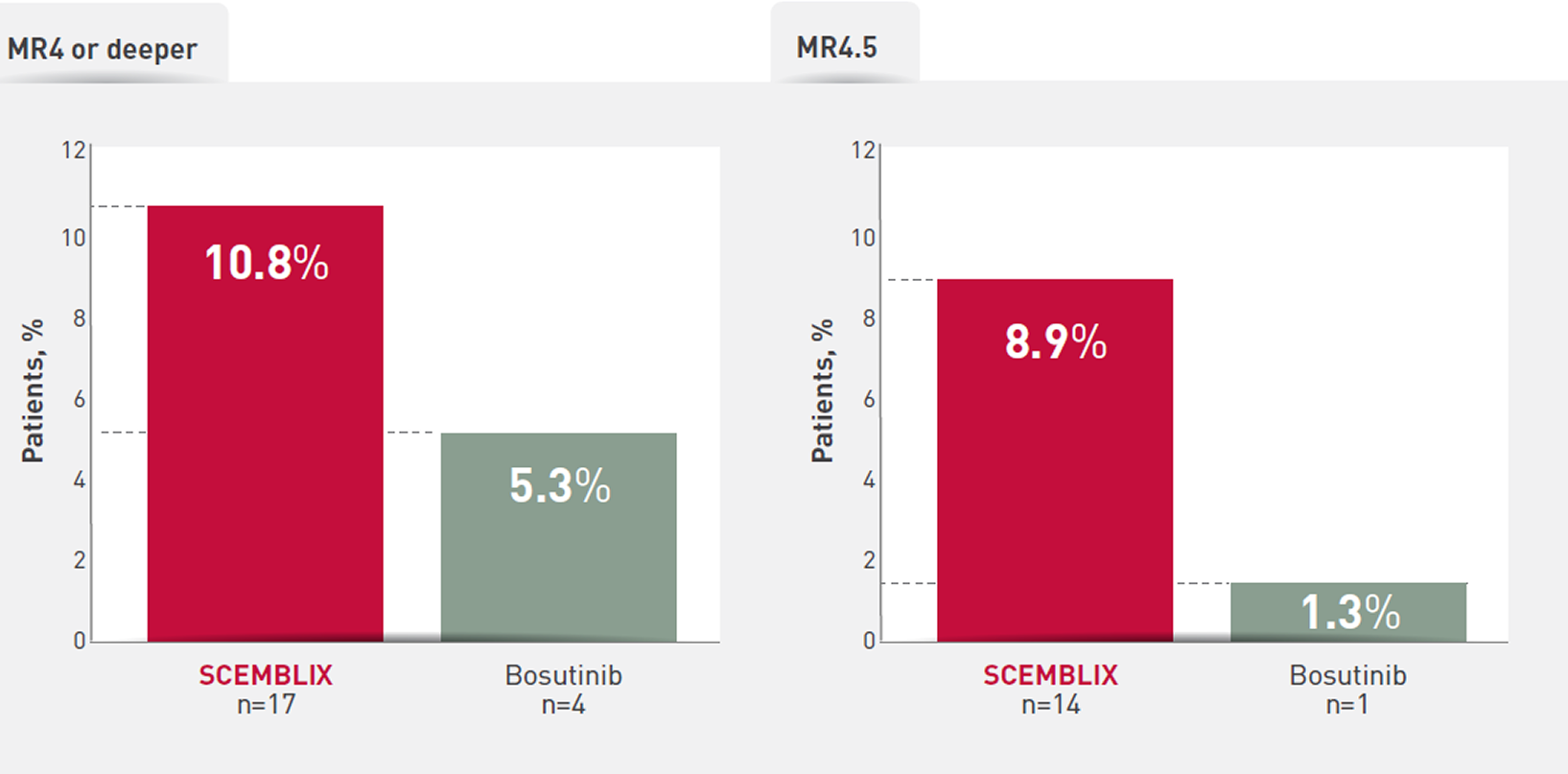

SCEMBLIX demonstrated a favourable safety profile vs bosutinib 1,6
AE, adverse event; CML, chronic myeloid leukaemia; MOA, mechanism of action; MMR, major molecular remission; Ph+ CML-CP, Philadelphia chromosome-positive chronic myeloid leukaemia in chronic phase; STAMP, Specifically Targeting the ABL1 Myristoyl Pocket; TKI, tyrosine kinase inhibitor; URTI, upper respiratory tract infection. CCyR, complete cytogenetic remission; CML-CP, chronic myeloid leukaemia in chronic phase; IS, international scale; MCyR, major cytogenetic response; MMR, major molecular response; STAMP, specifically targeting the ABL1 myristoyl pocket; TKI, tyrosine kinase.
SCEMBLIX is indicated for the treatment of patients 18 years of age and above with:
- Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP).
- Previously treated Ph+ CML in CP.
- Ph+ CML in CP with the T315I mutation
SCEMBLIX™ 20mg and 40mg NSS
SCEMBLIX™ 20mg and 40mg NSS
References
Réa D, Mauro MJ, Boquimpani C, et al. Blood. 2021 Nov 25;138(21):2031-2041.
Mauro MJ, Minami Y, Rea D et al. Presented at: American Society for Hematology 63rd Annual Meeting; December11-14, 2021 [Oral presentation 310].
Kantarjian HM, Giles FJ, Bhalla KN, et al. Blood 2011;117(4):1141-1145.
O'Brien SG, Guilhot F, Larson RA, et al. N Engl J Med. 2003;348(11):994-1004.
Saglio G. Kim D-W, Issaragrisil S. et al N Engl J Med.2010;362(24):2251-2259.
Kantarjian H, Shah NP. Hochhaus A, et al. N Engl J Med. 2010 ; 362(24):2260-2270.
Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. J Clin Oncol. 2018;36(3):231237.
Cortes JE, Kantarjian HM, Brümmerorf TH, et al. Blood 2011;118(17):4567-4576.
Shah NP. Kantarjian HM, Kin DW, et al. J Clin Oncol. 2008:26(19):3204-3212.
Data on file. ABL001A: ASCEMBL (A2301). Novartis Pharmaceuticals Corp; 2020.
Smith G, Apperley J, Milojkovic D, et al. Br J Haematol. 2020 Oct;191(2):171-193.
Hochhaus, A., Baccarani, M., Silver, R.T. et al. Leukemia. 2020;34:966–984.
SCEMBLIX (asciminib) approved Product Information.
