Hortobagyi GN, et al. Presented at the San Antonio Breast Cancer Symposium 2023, 5–9 December, San Antonio, Texas, USA.
Slamon DJ, et al. Ther Adv Med Oncol. 2023;15:1–16.
Slamon D, et al. LBA500 Presented at the American Society of Clinical Oncology 2023, 2–6 June, Chicago, USA.
Harbeck N, et al. Ann Oncol. 2021;32:1571–1581.
Fasching PA, et al. Oral LBA13. Presented at the European Society for Medical Oncology Congress 2024, 13–17 September, Barcelona, Spain.
Fasching PA, et al. Oral LBA13. Presented at the European Society for Medical Oncology Congress 2024, 13–17 September, Barcelona, Spain.
Harbeck N, et al. Nat Rev Dis Primers. 2019;5:66.
Hortobagyi GN, et al. Oral GS03-03. Presented at the San Antonio Breast Cancer Symposium 2023, 5–9 December, San Antonio, Texas, USA.
Fasching PA, et al. Oral LBA13. Presented at the European Society for Medical Oncology Congress 2024, 13–17 September, Barcelona, Spain.
Graff SL, et al. Poster PO1-17-07. Presented at the San Antonio Breast Cancer Symposium 2023, 5–9 December, San Antonio, Texas, USA.
Slamon D, et al. Abstract LBA500. Presented at the American Society of Clinical Oncology 2023, 2–6 June, Chicago, USA.
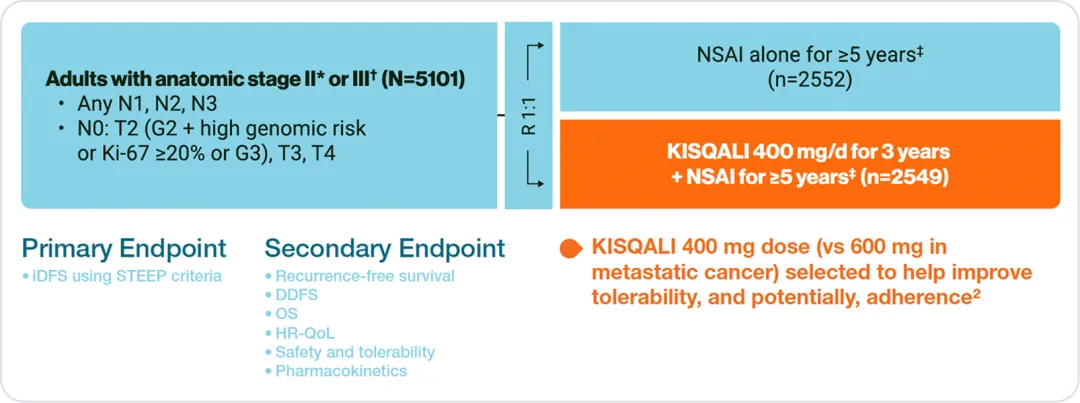
NATALEE included a wide range of patients with
HR+/HER2– eBC1,2
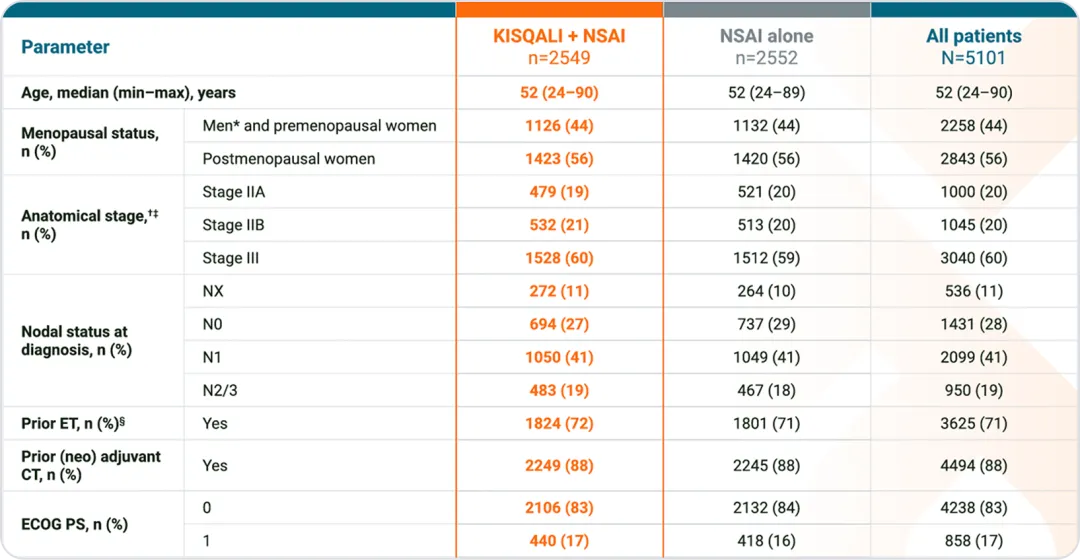
NATALEE baseline characteristics3
NATALEE population2
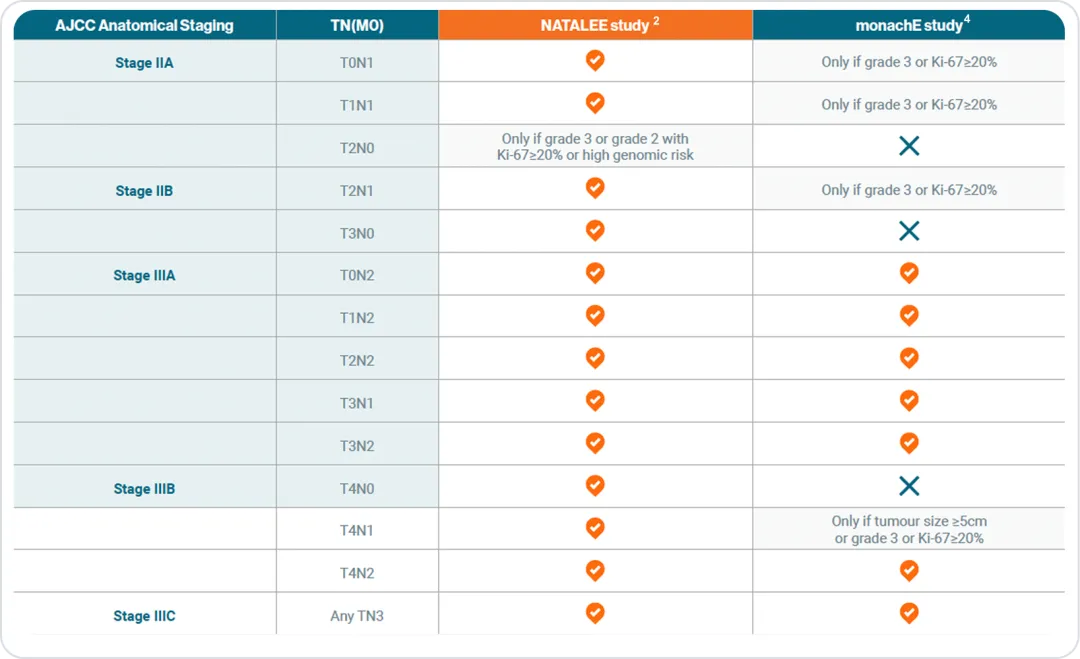
NATALEE included an expanded population of HR+ HER2- eBC patients compared to monachE.2 NATALEE included select N0 patients with high-risk features (stage IIA: Grade 2 with Ki-67 ≥20%, Oncotype DX ≥26, or high-risk genomic features, or grade 3; stage IIB/III: Any N0) and patients with any node positive disease (N1-N3), whereas monarchE excluded N0 and allowed only select N1 patients with additional high-risk criteria (grade 3, tumor ≥5 cm, or Ki-67 ≥20).2
References
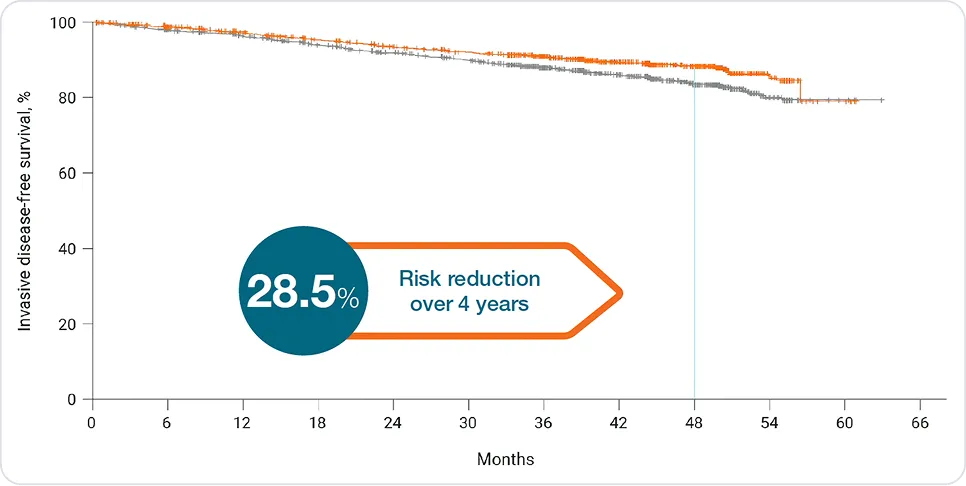
Invasive disease-free survival
In HR+/HER2– eBC:
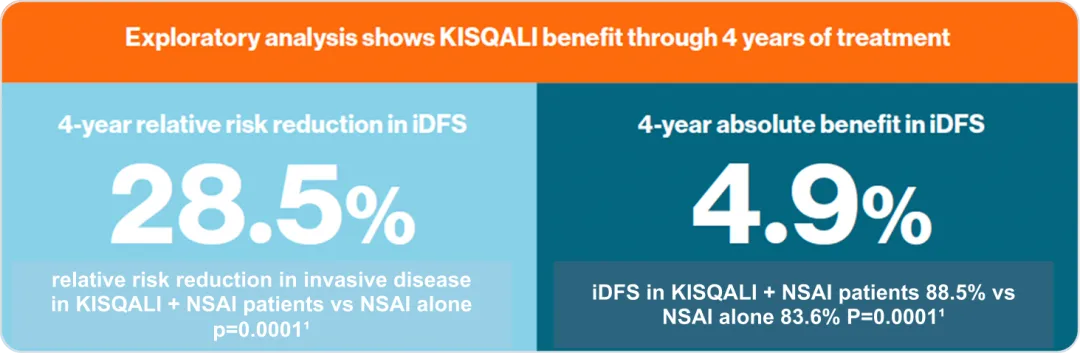
At 4 years, KISQALI® + NSAI prevented more than 1 in 4 recurrences vs. NSAI alone¹
In HR+/HER2– eBC:
At 4 years, KISQALI® + NSAI prevented more than 1 in 4 recurrences vs. NSAI alone¹
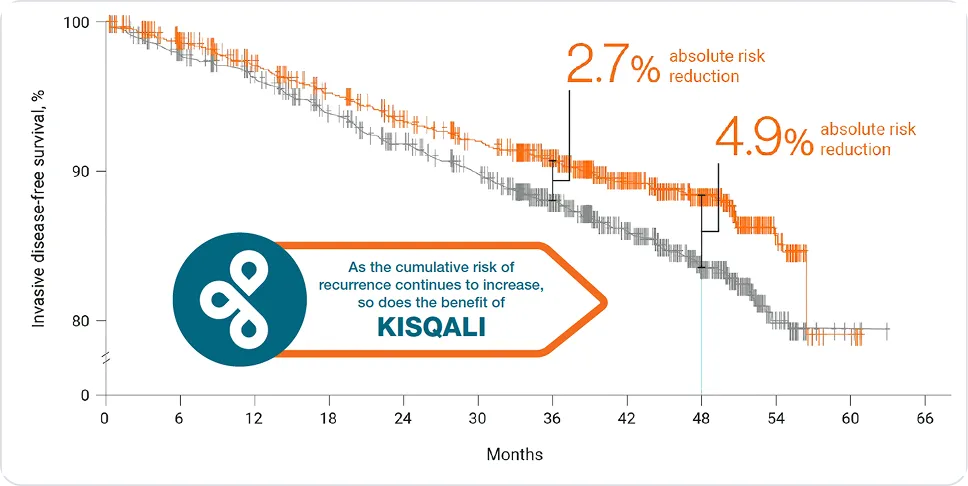
In HR+/HER2- eBC vs. NSAI alone:
KISQALI® can help you prevent more than 1 in 4 recurrences*1
Reference
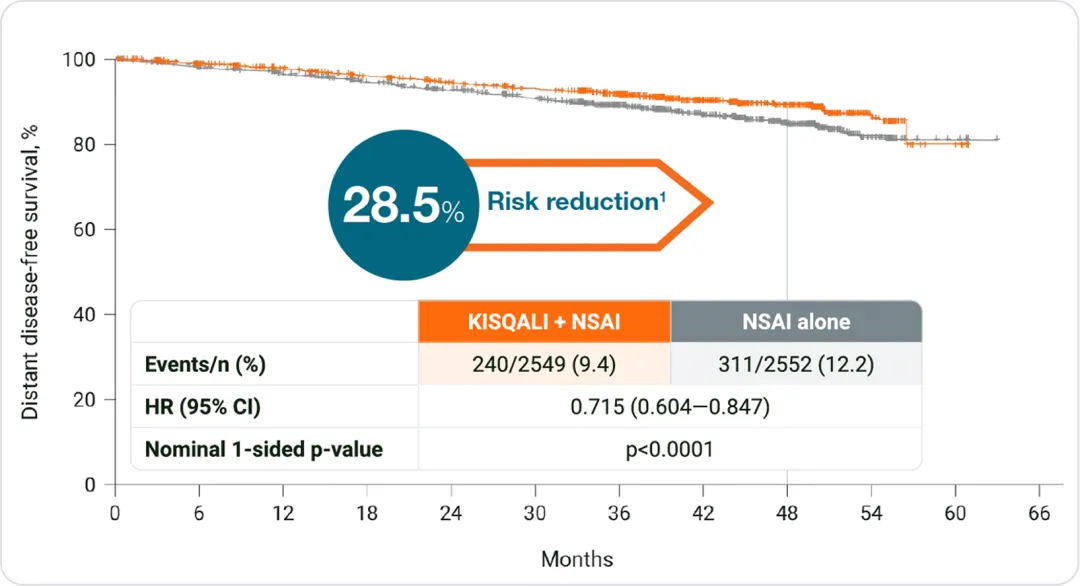
Distant disease-free survival
In HR+/HER2- eBC vs. NSAI alone:
At 4 years, KISQALI® + NSAI prevented more than 1 in 4 incurable metastatic recurrences*1,2
References
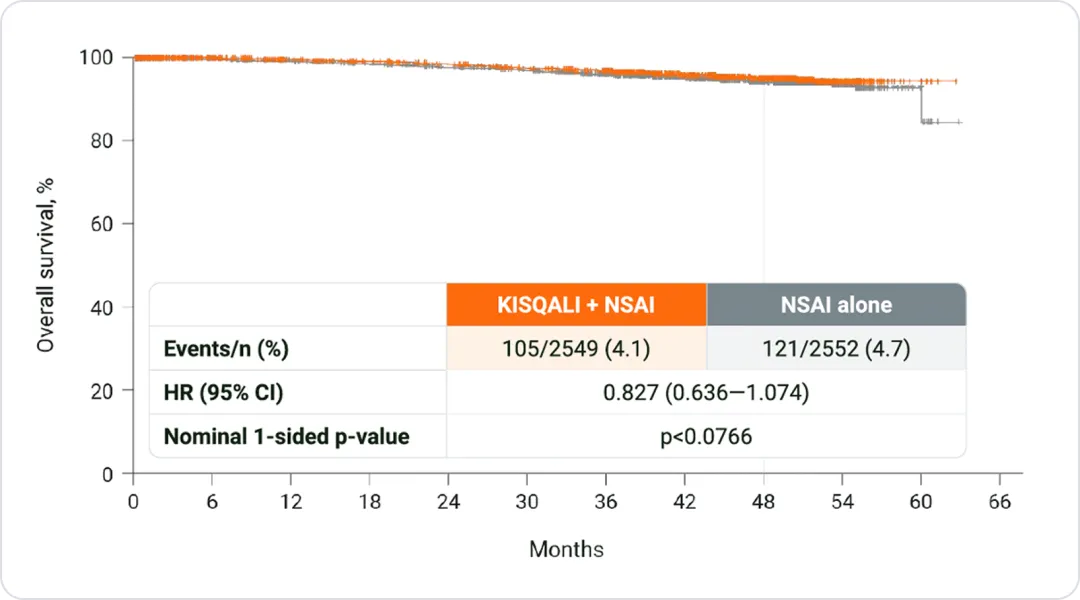
KISQALI® + NSAI showed a positive trend for OS at 4 years*¹
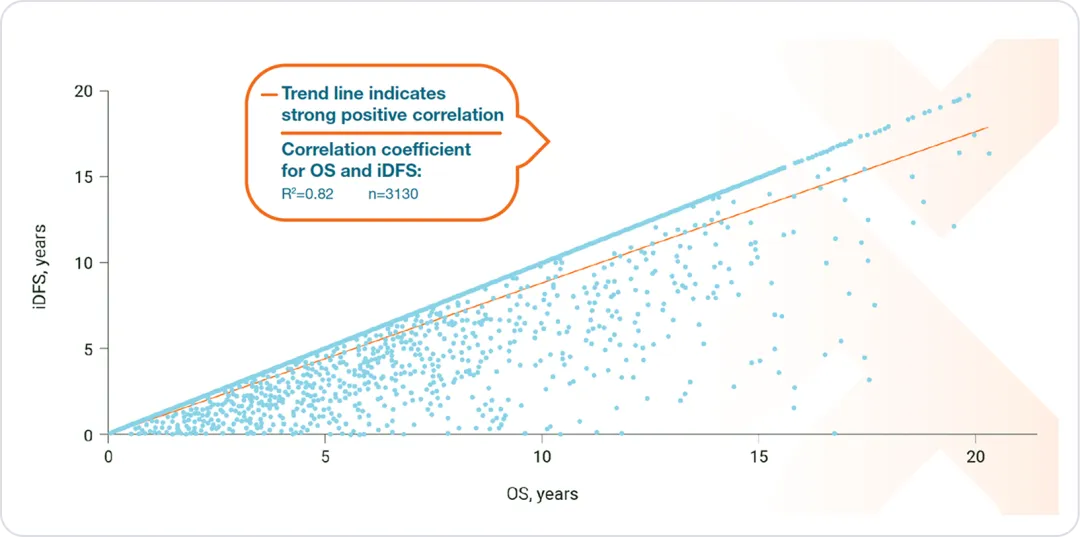
iDFS can act as a reliable surrogate endpoint for OS in HR+/HER2- eBC2
CI, confidence interval; eBC, early breast cancer; HER2-, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive; HR, hazard ratio; iDFS, invasive disease-free survival; NSAI, non-steroidal aromatase inhibitor; OS, overall survival; R2, coefficient of determination.
References
KISQALI® NSS - UAE
KISQALI® NSS - UAE