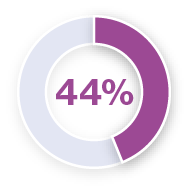TAFINLAR® (dabrafenib) + MEKINIST® (trametinib) dose modifications
TAFINLAR in combination with MEKINIST is indicated in adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.1,2
TAFINLAR in combination with MEKINIST is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.1,2
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
Helping patients on their TAFINLAR + MEKINIST treatment journey
Dose reduction, treatment interruption or treatment discontinuation may be required to manage adverse events (AEs) associated with TAFINLAR + MEKINIST treatment1,2
When a patient’s AEs are effectively managed, then a return to the starting dose, via dose escalation, can be considered in the same stepwise approach done for dose reductions1,2
Specific guidelines are available on the management of pyrexia, a common AE of TAFINLAR + MEKINIST treatment1,2
When discussing TAFINLAR + MEKINIST with your patients, it may be helpful to share key information on managing AEs associated with treatment.
Visit the section on dosing and administration for details on initiating and maintaining treatment, and on how to administer the combination.
AEs associated with TAFINLAR + MEKINIST are well established and are generally similar in both the metastatic and adjuvant settings3,4
Whilst TAFINLAR + MEKINIST is likely to cause patients to experience some AEs, it has a well-established side effect profile that is generally consistent across the metastatic and adjuvant settings.3,4
The management of AEs associated with TAFINLAR + MEKINIST treatment may require dose reduction, treatment interruption or treatment discontinuation.1,2
Dose modification schedule based on the grade of AEs excluding pyrexia1,2
Grade (CTC-AE)* | Recommended TAFINLAR dose modification | Recommended MEKINIST dose modification |
Grade 1 or Grade 2 (tolerable) | Continue treatment and monitor as clinically indicated | Continue treatment and monitor as clinically indicated |
Grade 2 (intolerable) or Grade 3 | Interrupt therapy until toxicity is Grade 0–1 and reduce by one dose level when resuming therapy | Interrupt therapy until toxicity is Grade 0–1 and reduce by one dose level when resuming therapy |
Grade 4 | Discontinue permanently, or interrupt therapy until Grade 0–1 and reduce by one dose level when resuming therapy | Discontinue permanently, or interrupt therapy until Grade 0–1 and reduce by one dose level when resuming therapy |
*The intensity of clinical adverse events graded by the Common Terminology Criteria for Adverse Events v4.0 (CTC-AE): Grade 1: Mild; intervention not indicated. Grade 2: Moderate; minimal, local or non-invasive intervention indicated. Grade 3: Severe or medically significant but not immediately life-threatening; hospitalisation or prolongation of hospitalisation indicated. Grade 4: Life-threatening consequences; urgent intervention indicated.
Recommended dose level reductions1,2
Dose level | TAFINLAR dose | MEKINIST dose |
Starting dose | 150 mg twice daily | 2 mg once daily |
1st dose reduction | 100 mg twice daily | 1.5 mg once daily |
2nd dose reduction | 75 mg twice daily | 1 mg once daily |
3rd dose reduction | 50 mg twice daily | 1 mg once daily |
| Dose adjustment of TAFINLAR <50 mg twice daily is not recommended | Dose adjustment of MEKINIST <1 mg once daily is not recommended |
Exceptions where dose modifications are necessary for only one of the two treatments are detailed below for uveitis, RAS mutation positive non-cutaneous malignancies (primarily related to dabrafenib), left ventricular ejection fraction (LVEF) reduction, retinal vein occlusion (RVO), retinal pigment epithelial detachment (RPED) and interstitial lung disease (ILD)/pneumonitis (primarily related to TAFINLAR + MEKINIST).
Dose modification for only one of the two treatments
TAFINLAR and MEKINIST should be simultaneously dose reduced, interrupted or discontinued. Exceptions, where dose modifications are necessary for only one of the two treatments, are shown in the table below.1,2
Cases where dose modifications are necessary for only one of the two treatments1,2
Modification of TAFINLAR dose only | Modification of MEKINIST dose only |
Uveitis: If uveitis does not respond to local ocular therapy, dabrafenib should be withheld until resolution of ocular inflammation and then dabrafenib should be restarted reduced by one dose level. RAS-associated non cutaneous malignancies: The benefits and risks should be considered before continuing treatment with TAFINLAR in patients with a non-cutaneous malignancy that has a RAS mutation. | RVO and RPED: In patients who are diagnosed with RVO, treatment with MEKINIST should be permanently discontinued. LVEF reduction: MEKINIST should be interrupted in patients who have an asymptomatic, absolute decrease of >10% in LVEF compared to baseline and the ejection fraction is below the institution's lower limit of normal (LLN). If the LVEF recovers, treatment with MEKINIST may be restarted, but the dose should be reduced by one dose level with careful monitoring. MEKINIST should be permanently discontinued in patients with Grade 3 or 4 left ventricular cardiac dysfunction or clinically significant LVEF reduction that does not recover within 4 weeks. Interstitial lung disease (ILD)/pneumonitis: MEKINIST must be withheld in patients with suspected ILD or pneumonitis, including patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnoea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations. MEKINIST must be permanently discontinued in patients diagnosed with treatment-related ILD or pneumonitis. |
Dose modifications or interruptions are not recommended for incidences of cutaneous squamous-cell carcinoma or new primary melanoma.1
When a patient’s side effects are effectively managed, then a return to the starting dose via dose re-escalation can be considered in the same stepwise approach as for dose reductions.1,2
Dose modifications in special populations1,2

Children and adolescents (<18 years)
The safety and efficacy of TAFINLAR + MEKINIST have not yet been established in children and adolescents (<18 years)

Elderly
No adjustment of the initial dose of TAFINLAR or MEKINIST is required in patients >65 years of age
More frequent dose adjustments of MEKINIST may be required in patients >65 years of age
Renal impairment
Mild to moderate: No dose adjustment of TAFINLAR or MEKINIST is required
Severe: Use with caution when administered as monotherapy or in combination therapy. There are no clinical data for TAFINLAR or MEKINIST in patients with severe renal impairment and the potential need for dose adjustment cannot be determined

Hepatic impairment
Mild: No dose adjustment of TAFINLAR or MEKINIST is required
Moderate to severe: TAFINLAR + MEKINIST should be used with caution in patients with moderate or severe hepatic impairment when administered as monotherapy or in combination
Pyrexia was the most common AE observed in Phase II and III clinical trials of TAFINLAR + MEKINIST.3–6
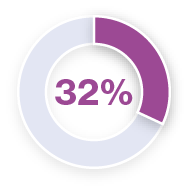
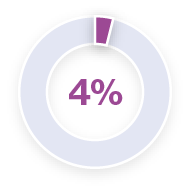
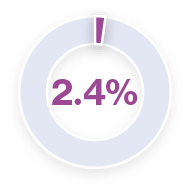
Approximately 50% of first occurrences happen in the first month of therapy and the majority of cases are mild to moderate.1,5,6 In the metastatic melanoma setting, the median duration of the first pyrexia event was three days (COMBI-d trial).7 In the adjuvant setting, the median duration of the first pyrexia event was two days (COMBI-APlus trial).6
During clinical trials, the majority of pyrexia events were mild or moderate (Grade 1 or 2).*3–6
Analysis of pyrexia in patients treated with TAFINLAR + MEKINIST in the COMBI-APlus clinical trial†3–6
*Pyrexia was analysed from the following clinical trials in melanoma and in metastatic non-small cell lung cancer (NSCLC): Registrational Phase II trial (NCT01336634) in metastatic NSCLC (n=82); COMBI-AD in resected Stage III melanoma (n=435); COMBI-d in unresectable or metastatic melanoma (n=209); COMBI-v in unresectable or metastatic melanoma (n=350).5 Pyrexia was also analysed in the primary analysis of the COMBI-APlus trial (NCT03551626) in resected Stage III melanoma (n=552).6
†COMBI-APlus is an open-label, Phase IIIb trial evaluating an adapted pyrexia management algorithm in patients with high-risk resected Stage III BRAF V600E/K–mutant melanoma treated with up to 12 months of adjuvant TAFINLAR + MEKINIST.6
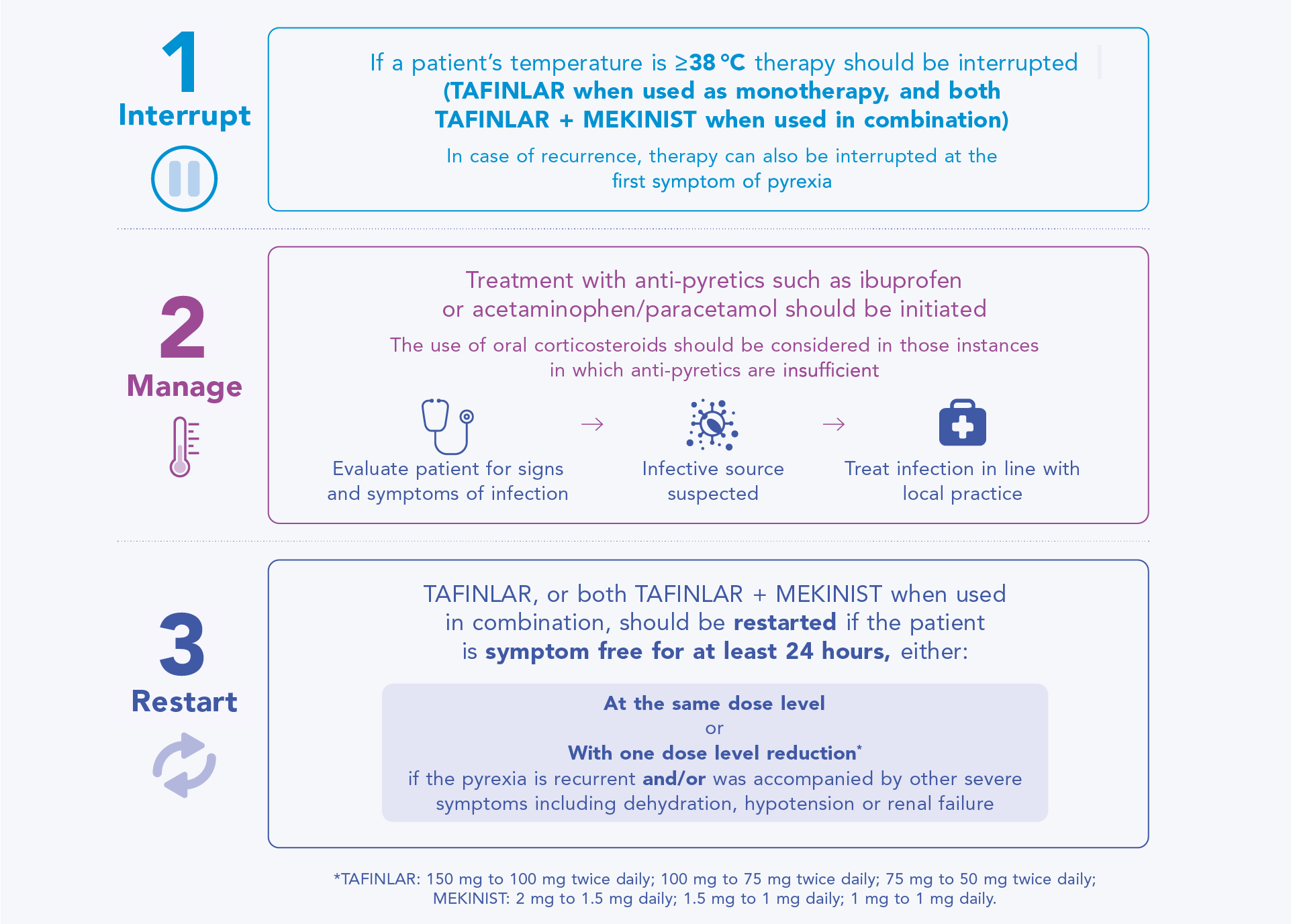
Guidance for managing pyrexia in patients receiving TAFINLAR + MEKINIST
Patients may respond well to dose interruption (TAFINLAR when used as monotherapy, and both TAFINLAR + MEKINIST when used in combination) and supportive care for management of pyrexia, allowing later re-initiation of treatment.1,2,6
The diagram below outlines the recommended approach to managing a pyrexia event that the healthcare team who manages a patient’s care should be aware of.
Management of pyrexia: interrupt, manage, restart1,2,6,8,9
In patients with melanoma, AEs in the adjuvant setting (COMBI-AD trial) and metastatic setting (COMBI-v and COMBI-d trials)4,5 were similar, and the most common AEs observed with TAFINLAR + MEKINIST in Phase III studies (occurring with an all-Grades incidence of ≥20%) included: pyrexia, chills, fatigue, headache, nausea, vomiting, diarrhoea, arthralgia and rash.4,5
Further information on managing side effects
For more information on potential AEs and how to manage them in patients receiving TAFINLAR + MEKINIST, please refer to the TAFINLAR + MEKINIST SmPCs.1,2
Always encourage your patients to carefully read the Patient Information Leaflets (PILs) in their TAFINLAR and MEKINIST packs before starting treatment, as they contain important information for them.1,2
Additional information is also available from your Novartis representative who can provide you with a comprehensive healthcare professional (HCP) brochure about TAFINLAR + MEKINIST.
AE, adverse event; BRAF V600, mutation of the BRAF gene in which valine (V) is substituted at amino acid 600; BRAF V600E/K, mutation of the BRAF gene at valine (V) 600 to either glutamate (E) or lysine (K); CTC-AE, Common Terminology Criteria for Adverse Events; HCP, healthcare professional; ILD, interstitial lung disease; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; NSCLC, non-small cell lung cancer; PIL, patient information leaflet; RPED, retinal pigment epithelial detachment; RVO, retinal vein occlusion; SmPC, summary of product characteristics.
AE, adverse event; BRAF V600, mutation of the BRAF gene in which valine (V) is substituted at amino acid 600; BRAF V600E/K, mutation of the BRAF gene at valine (V) 600 to either glutamate (E) or lysine (K); CTC-AE, Common Terminology Criteria for Adverse Events; HCP, healthcare professional; ILD, interstitial lung disease; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; NSCLC, non-small cell lung cancer; PIL, patient information leaflet; RPED, retinal pigment epithelial detachment; RVO, retinal vein occlusion; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
Robert C, et al. N Engl J Med 2019;381:626–636.
Long GV, et al. N Engl J Med 2017;377:1813–1823.
Robert C, et al. Presented at ESMO 2019;27 September – 1 October, Barcelona, Spain.
Atkinson V, et al. Poster 9525. Presented at ASCO 2021.
Long GV, et al. N Engl J Med 2014;371:1877–1888.
Welsh SJ, Corrie PG. Ther Adv Med Oncol 2015;1–15.
Common Terminology Criteria for Adverse Events (CTCAE) V4.0.
UK | March 2025 | FA-11220698
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.