

Mechanisms of action of TAFINLAR® (dabrafenib) + MEKINIST® (trametinib)
TAFINLAR in combination with MEKINIST is indicated in adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.1,2
TAFINLAR in combination with MEKINIST is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.1,2
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
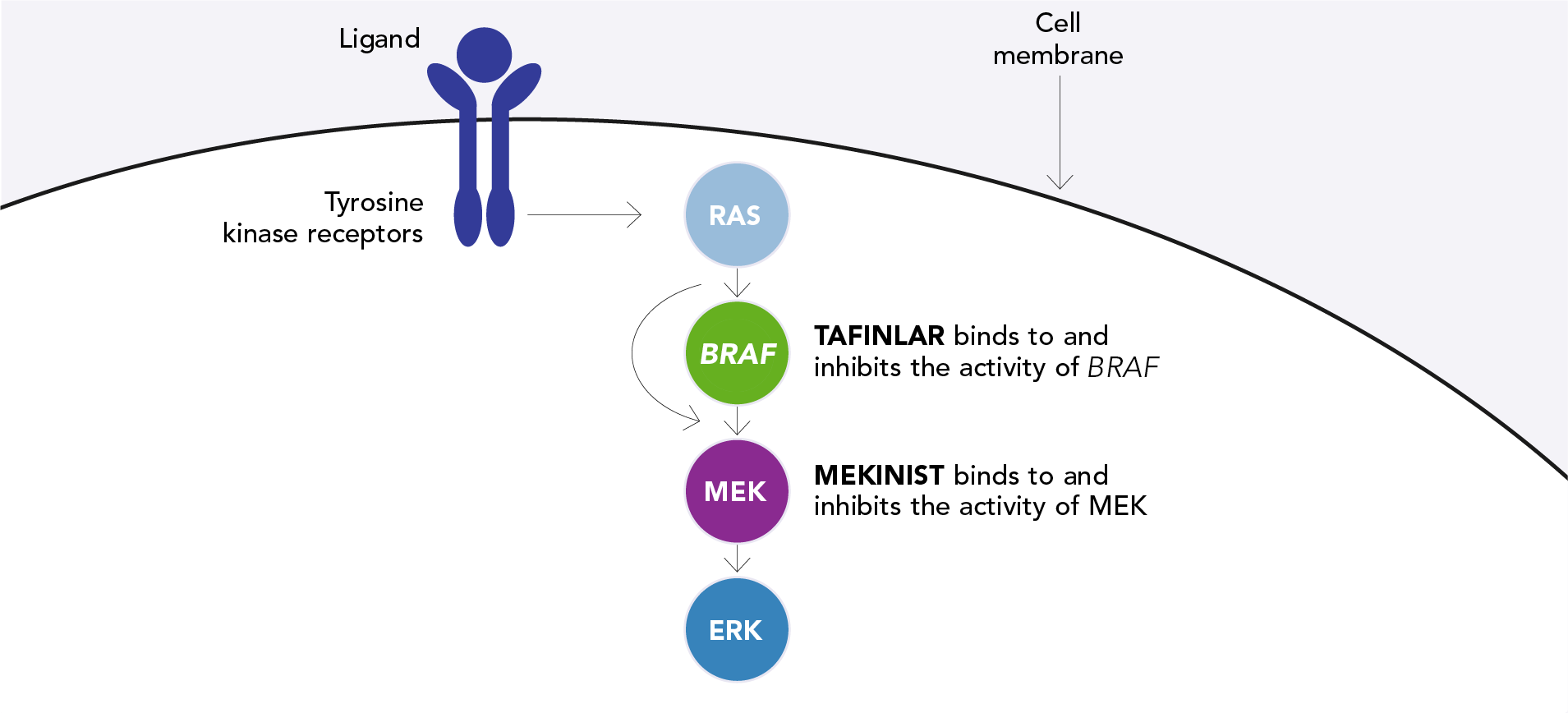
TAFINLAR + MEKINIST target two distinct points on the mitogen-activated protein kinase (MAPK) pathway to provide concomitant inhibition1,2
BRAF V600 mutations result in constitutive activation of the MAPK pathway, which plays a key role in regulating the growth, proliferation and survival of normal cells, including melanocytes, which are the cells from which melanoma originates.3,4 As many as 50% of patients with melanoma harbour mutations of the BRAF V600 gene.5
In melanoma cells with a BRAF V600 mutation, the BRAF V600 and mitogen-activated extracellular signal-regulated kinase (MEK) proteins send signals that cause melanoma cells to grow and increase uncontrollably. TAFINLAR + MEKINIST work together to block these signals.1,2
TAFINLAR, in combination with MEKINIST, is designed to target the oncogenic driver of BRAF V600-positive melanoma. TAFINLAR is an inhibitor of the mutated BRAF V600 kinase1 and MEKINIST is a reversible and highly selective inhibitor of MEK 1 and MEK 2, which sit downstream of BRAF.2 Based on pre-clinical research, combined inhibition of BRAF and MEK reduces extracellular signal-related kinase (ERK)-driven gene expression that can cause tumour growth.
Schematic of the MAPK signalling pathway showing where TAFINLAR and
MEKINIST act1,2
Further information about BRAF V600 mutations, the BRAF pathway, and the importance of BRAF testing in melanoma can also be found here.
Benefits of blocking MEK, in addition to BRAF
Combining TAFINLAR + MEKINIST results in greater inhibition of tumour growth versus either drug alone in BRAF V600-positive melanoma1,2
Blocking MEK, in addition to BRAF in BRAF V600-positive melanoma:
Prolonged inhibition of tumour growth with TAFINLAR + MEKINIST vs TAFINLAR alone (median progression-free survival was 11.0 vs 8.8 months, respectively [hazard ratio=0.67; 95% CI: 0.53–0.84, p= 0.0004])6
Is thought to reduce the risk of treatment resistance vs BRAF inhibitor alone, based on pre-clinical research1–3,7–10
Cutaneous malignancies are a special warning associated with TAFINLAR + MEKINIST. Please refer to the SmPC for the full guidance.
BRAF V600, mutation of the BRAF gene in which valine (V) at amino acid 600; CI, confidence interval; ERK, extracellular signal-related kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated extracellular signal-regulated kinase; RAS, rat sarcoma; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
Nijenhuis CM, et al. Cancer Treat Rev 2013;39:305–312.
Melanoma UK. What is melanoma? Available at: https://www.melanomauk.org.uk/pages/category/what-is-melanoma [Accessed March 2025].
Ascierto PA, et al. J Transl Med 2012;10:85.
Long GV, et al. Ann Oncol 2017;28(7):1631–1639.
Hauschild A, et al. J Clin Oncol 2013;31(suppl): abstract number 9013.
Robert C, et al. Pigment Cell Melanoma Res 2018;31:201.
McArthur GA, et al. Lancet Oncol 2014; 15: 323–332.
Greger JG, et al. Mol Cancer Ther 2012;11:909–920.
UK | April 2025 | FA-11220701-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.