

Patient-reported outcome measures in CSU
Indications:1
Xolair is indicated as add-on therapy for the treatment of chronic spontaneous urticaria (CSU) in adult and adolescent (12 years and above) patients with inadequate response to H1 antihistamine treatment.
The recommended dose of Xolair in patients with chronic spontaneous urticaria is 300 mg by subcutaneous injection every four weeks.1
Please refer to the Xolair Summary of Product Characteristics (SmPC) for the full therapeutic indication.1
Disease control and symptom control may be a possibility with Xolair2,3
At Week 12, the mean change from baseline in weekly ISS (baseline: 14.0 ± 3.6) was −8.6 (95% CI, −9.3 to −7.8) in the Xolair group vs −4.0 (95% CI, −5.3 to 2.7) in the placebo group (baseline: 13.8 ± 3.6) (p<0.001).3
At Week 12, the mean change from baseline in UAS7 (baseline: 31.2 ± 6.6) was −19.0 (95% CI, −20.6 to −17.4) in the Xolair group vs −8.5 (95% CI, −11.1 to −5.9) in the placebo group (baseline: 30.2 ± 6.7) (p<0.001).3
The 2021 international EAACI/GA2LEN/EuroGuiDerm/APAAACI guidelines state that the “goal of treatment is to treat the disease until it is gone and as efficiently as possible, aiming at a continuous UAS7=0, complete control and a normalisation of quality of life.”4
As recommended by the latest CSU treatment guidelines, patient-reported outcome measures (PROMs) are crucial to evaluating and monitoring disease activity and severity, disease control and quality of life in patients with urticaria.4,5
See below to learn more about each key PROM for CSU and either access editable PDF downloads or submit a contact form to order complimentary hard copies from Novartis.
The UCT is designed to assess urticaria control. It is simple to complete and is suited to tracking every 4 weeks, although a UCT-7 is also available which tracks urticaria control every 7 days.4,5
Validated and specifically designed for chronic urticaria
Self-administered, retrospective (past 4 weeks) 4-item questionnaire
Each answer is scored ranging from 0 to 4
The summed score ranges from 0 (no control) to 16 (complete control)
The UCT covers the following 4 domains5

Overall CSU control

Physical symptoms (itch, hives, swelling)

Quality of life impact

Frequency of treatment inadequacy
UCT score interpretation by the 2021 EAACI guidelines4
Minimal important clinical difference: 3 points5
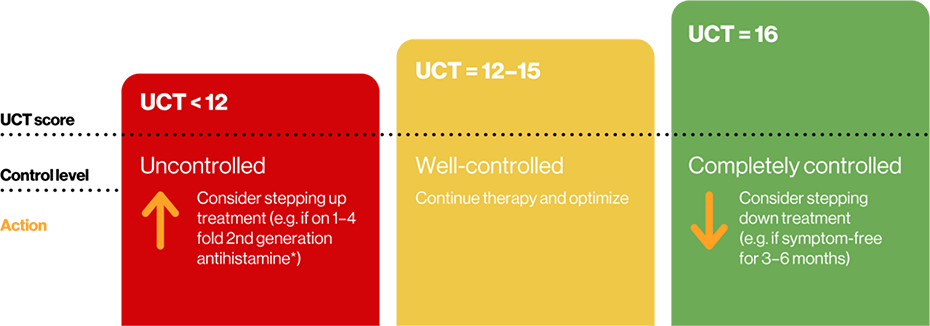
Adapted from Zuberbier T, et al.4
*Novartis does not condone the off label use of medicines. Please refer to individual products’ Summary of Product Characteristics before prescribing.
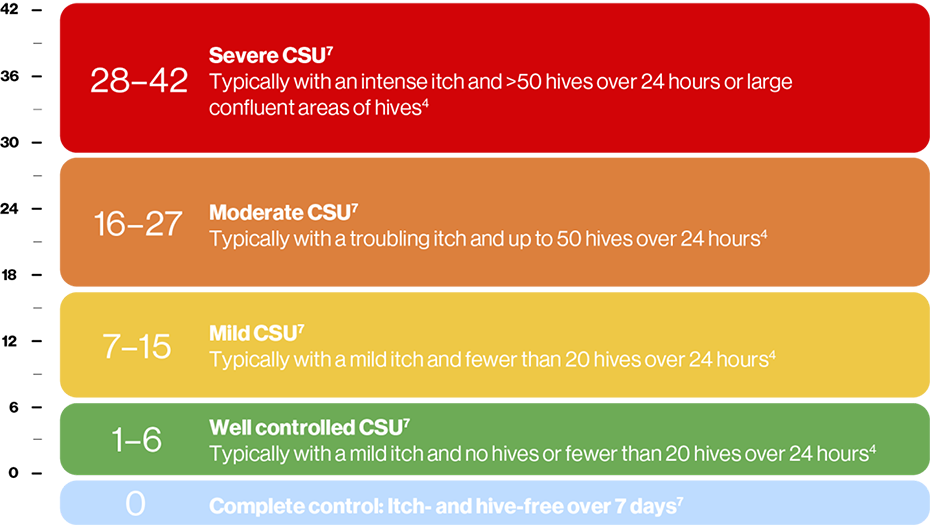
The UAS7 is designed to assess disease activity and severity.6 It is a comprehensive measure that requires the patient to record their symptoms daily for a week. The UAS7 should be used in routine clinical practice to determine disease activity and response to treatment of patients with CSU. Scores are summarised over one week (7 days) for a maximum of 42 weeks.4
Patient scores the intensity of their hives (number of and area covered by wheals [0 to 3] and itch [0 to 3]) each day for 7 days, where 0=none, 1=mild, 2=moderate and 3=intense4
Maximum weekly score is 42 – a higher score corresponds to more severe disease5
Severe CSU has a UAS7 score of 28–427
Included in the NICE guidance as an example of objective assessment of disease severity8
The UAS7 does not assess angioedema. Patients with angioedema should use the Angioedema Activity Score (AAS)4
Here is a figure to explain how UAS7 scoring can be interpreted:
The DLQI is a simple, weekly, validated questionnaire used to evaluate health-related quality of life in patients with skin diseases – not only CSU.9 Where patients may experience comorbid conditions, it may also be useful to consider other specific QoL measures such as the Cu-Q2oL, which assesses itch, hives and angioedema, and AE-QoL, which assesses angioedema alone.10 (Both not available here due to copyright).
The DLQI consists of:9

Symptoms and feelings

Daily activities

Personal relationships

Leisure

Work and school

Treatment
The effect of the disease on each domain is scored from 0 (not at all) to 3 (very much) for each question, giving a total score of between 0 and 309
How scores can be interpreted:11
0–1 no effect at all on patient’s life
2–5 small effect on patient’s life
6–10 moderate effect on patient’s life
11–20 large effect on patient’s life
21–30 extremely large effect on patient’s life
Patient-reported outcomes resources
Click on the links below to view the patient-reported outcomes resources, and if you have any further requests, please contact a Novartis representative.

Symptom control
Four questions
Quick
Retrospective (4 weeks)
Use at first consultation and every follow-up consultation

Disease severity
Two questions
Symptoms scored daily
Prospective (1 week)
Use at every follow-up consultationt

Quality of life
10 questions
Not specific to CSU
Retrospective (1 week)
Use at first consultation and every 6 months thereafter
As part of our mission to be as environmentally friendly as possible, we provide our resources in electronic form.
These materials should not be printed by healthcare professionals.
AE-QoL, angioedema-quality of life questionnaire; BSACI, British Society for Allergy and Clinical Immunology; CI, confidence interval; Cu-Q2oL, chronic urticaria-quality of life questionnaire; CSU, chronic spontaneous urticaria; DLQI, dermatology life quality index; EAACI, European Academy of Allergy and Clinical Immunology; EDF, European Dermatology Forum; GA2LEN, Global Allergy and Asthma European Network; IgE, immunoglobulin E; ISS, itch severity score; NICE, National Institute for Health and Care Excellence; PROM, patient-reported outcome measure; SmPC, summary of product characteristics; UAS7, urticaria activity score at seven days; UCT, urticaria control test; WAO, World Allergy Organization.
References
Xolair® (omalizumab) Summary of Product Characteristics.
Novartis Data on File. XSU15-R002. Proportion of UAS7 MID responders at Week 12 – GLACIAL study.
Kaplan A, et al. J Allergy Clin Immunol 2013;132(1):101–109.
Zuberbier T, et al. Allergy 2022;77(3):734–766.
Moestrup K, et al. Int J Dermatol 2017;56(12):1342–1348.
Sabroe RA, et al. Br J Dermatol 2022;186(3):398–413.
Stull D, et al. Br J Dermatol 2017;177:1093–1101.
National Institute for Health and Care Excellence. How should I diagnose urticaria? Available at: https://cks.nice.org.uk/topics/urticaria/diagnosis/diagnosis/ [Accessed March 2025].
Finlay AY, et al. Clin Exp Dermatol 1994;19(3):210–216.
Weller K , et at. J Eur Acad Dermatol Venereol 2015;29 (Suppl. 3);38–44.
Cardiff University School of Medicine. Dermatology Life Quality Index. Available at: https://www.cardiff.ac.uk/medicine/resources/quality-of-life-questionnai... [Accessed March 2025].
Weller K, et al. J Allergy Clin Immunol 2014;133(5):1365–1372.
UK | March 2025 | FA-11360062
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.
