
Aimovig® (erenumab)
Zdravilo Aimovig® (erenumab) je indicirano za profilakso migrene pri odraslih, ki imajo najmanj 4 migrenske dni na mesec.1

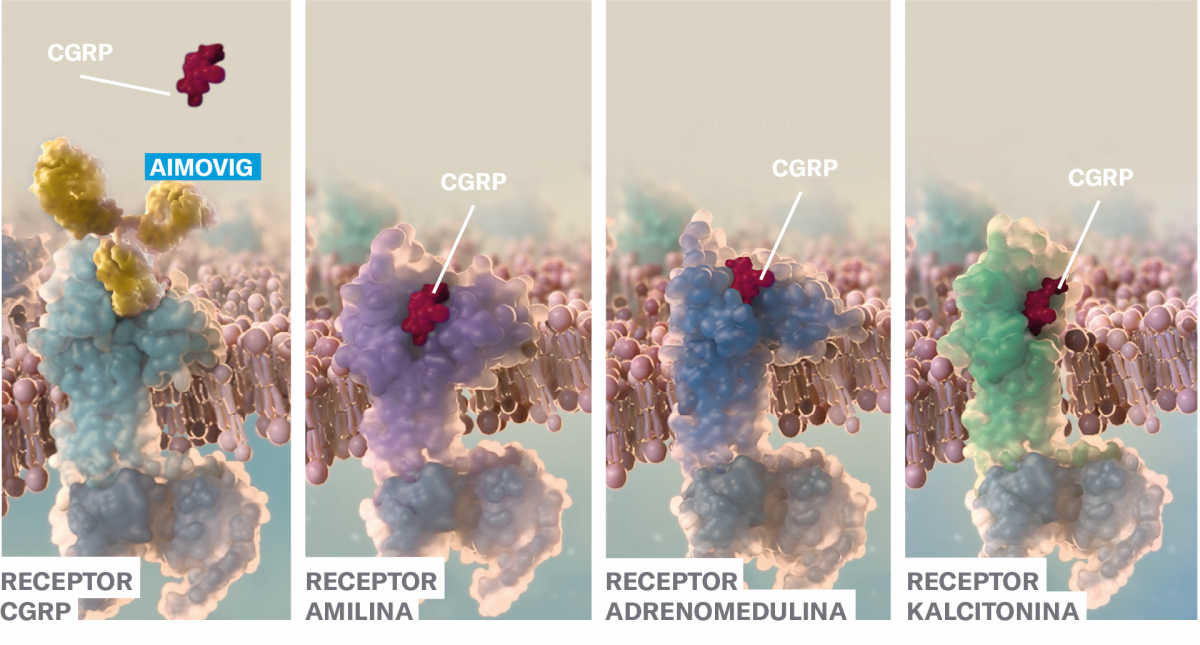
Prvo in edino popolnoma humano monoklonsko protitelo, ki se ciljano in z visoko afiniteto veže na receptor za CGRP.2, 3
Z dolgoročnimi dokazi, za manj migrenskih dni.6
Vrednotenje varnostnega profila je trajalo do 5 let: najdaljša študija zdravila anti-CGRP.6
Profil varnosti in prenosljivosti je podoben kot pri placebu.4,5,6
Enostavno odmerjanje enkrat na 4 tedne.1
35,5 % bolnikov z epizodno migreno je bilo po 5 letih povsem brez migrenskih napadov.6

Reference:
Povzetek glavnih značilnosti zdravila Aimovig®. Junij 2023.
Shi L, Lehto SG, Zhu DXD et al. Pharmacologic Characterization of AMG 334, a Potent and Selective Human Monoclonal Antibody against the Calcitonin Gene-Related Peptide Receptor. J Pharmacol Exp Ther 2016; 356:223–231.
Russo AF. Calcitonin Gene-Related Peptide (CGRP): A New Target for Migraine. Annu. Rev. Pharmacol. Toxicol 2015. 55:533–52.
Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132.
Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
Ashina M et al. Long-term effi cacy and safety of erenumab in migraine prevention: results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur J Neurol. 2021. doi: 10.1111/ene.14715.
Novartis Fourth Quarter and Full year 2020 Condensed interim financial report – Supplementary Data.
FA-11532804

