
Xolair (Omalizumab) is a recombinant DNA-derived humanized monoclonal antibody that selectively binds to human immunoglobulin E (IgE)1
Xolair is indicated as add-on therapy:
For the treatment of chronic spontaneous urticaria in adult and adolescent (12 years and above) patients with inadequate response to H1 antihistamine treatment1
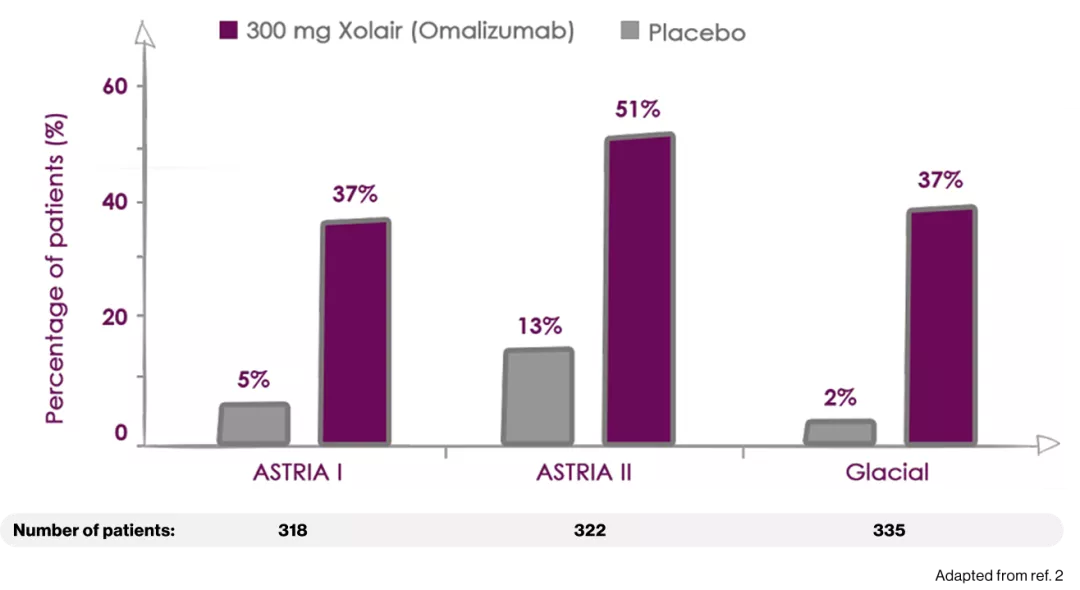
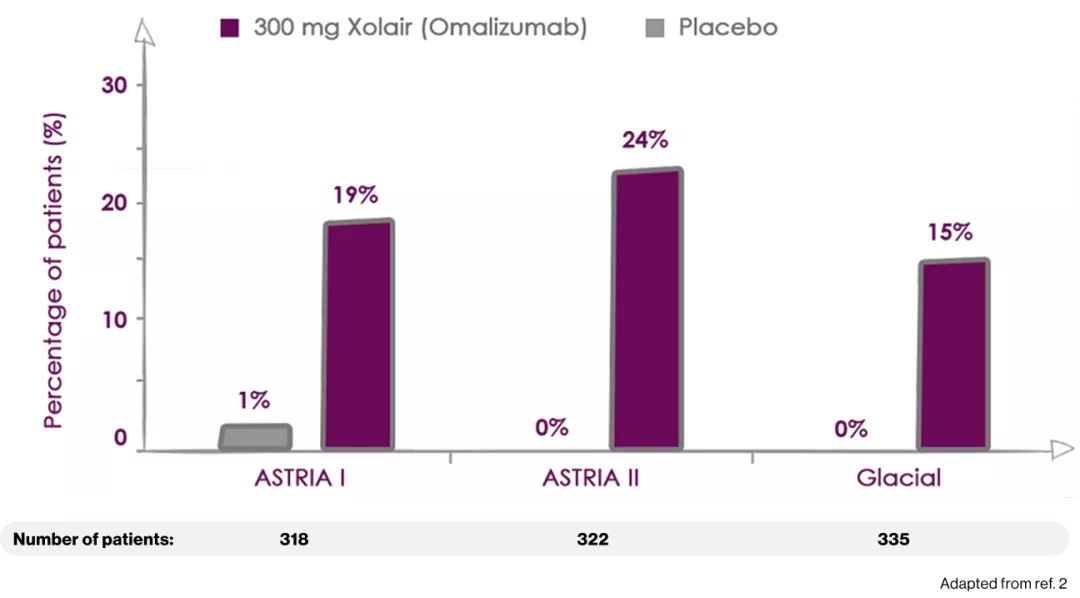
The 300-mg dose of Xolair (omalizumab) produced the HIGHEST response rates among all study arms2
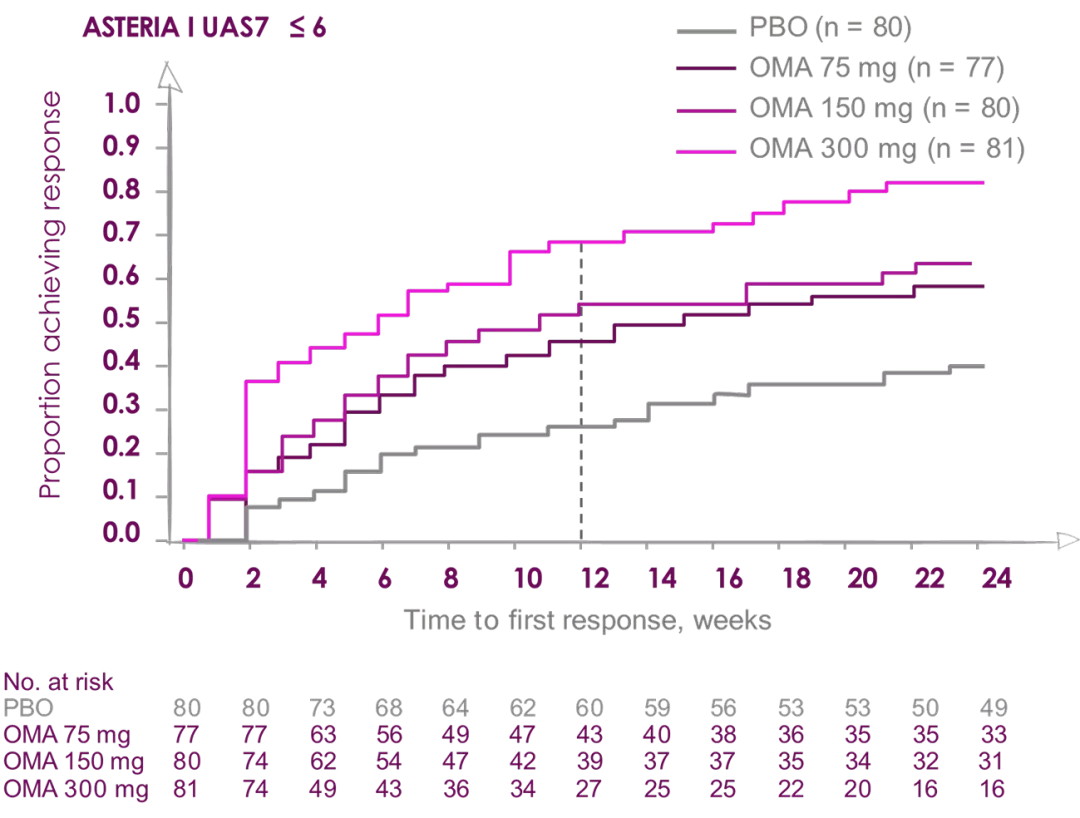
Percentage of patients reporting WELL-CONTROLLED Urticaria (UAS7 ≤6)
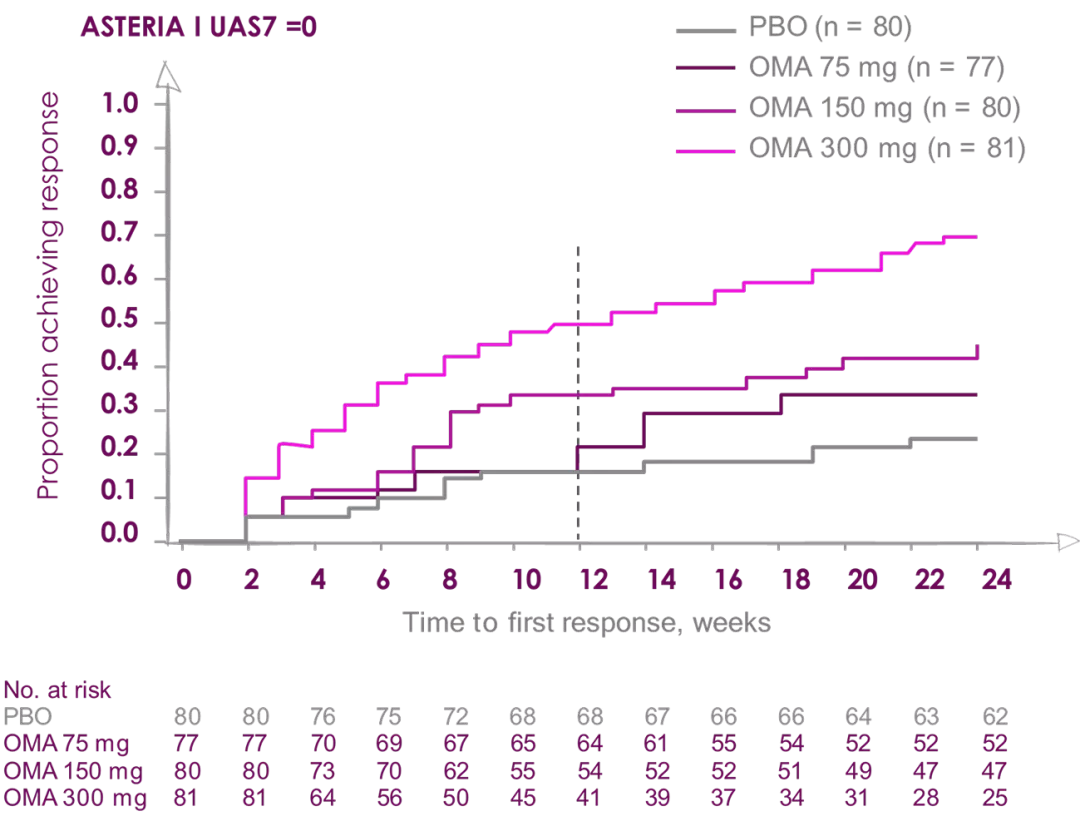
Percentage of patients reporting COMPLETE RESPONSE (UAS7=0)
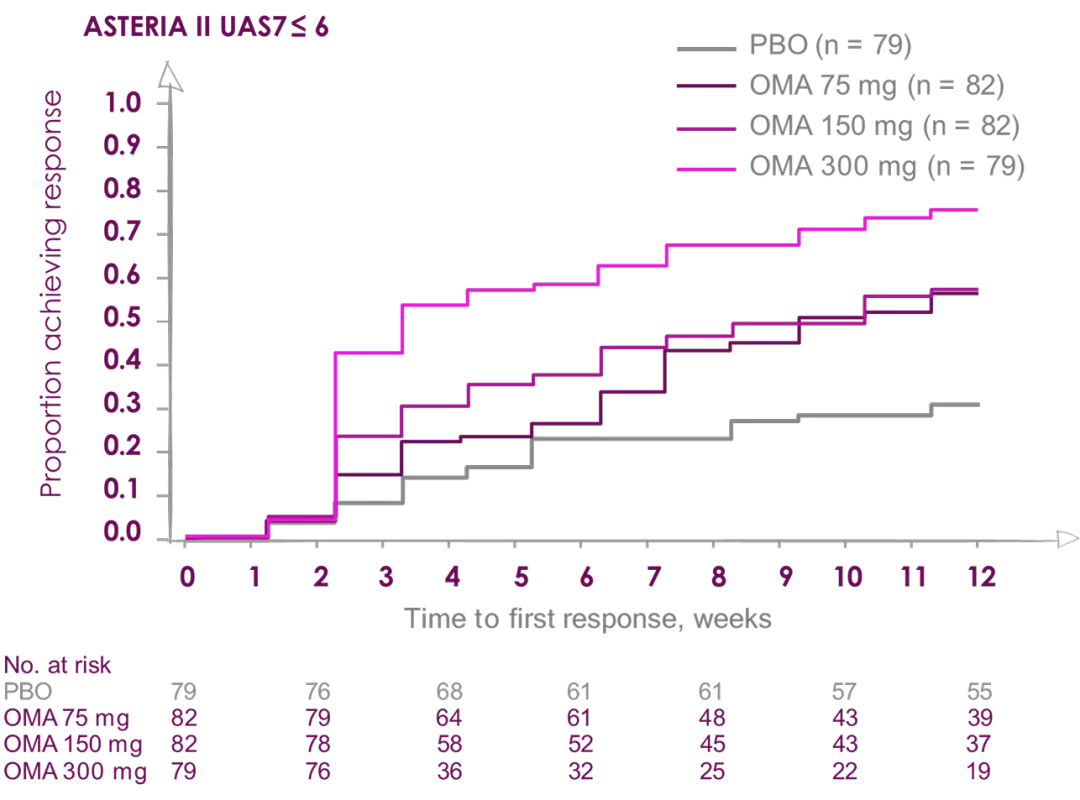
Median time to achieve well-controlled urticaria (UAS7 ≤6) was
3 weeks in patients receiving Xolair (Omalizumab) 300 mg2
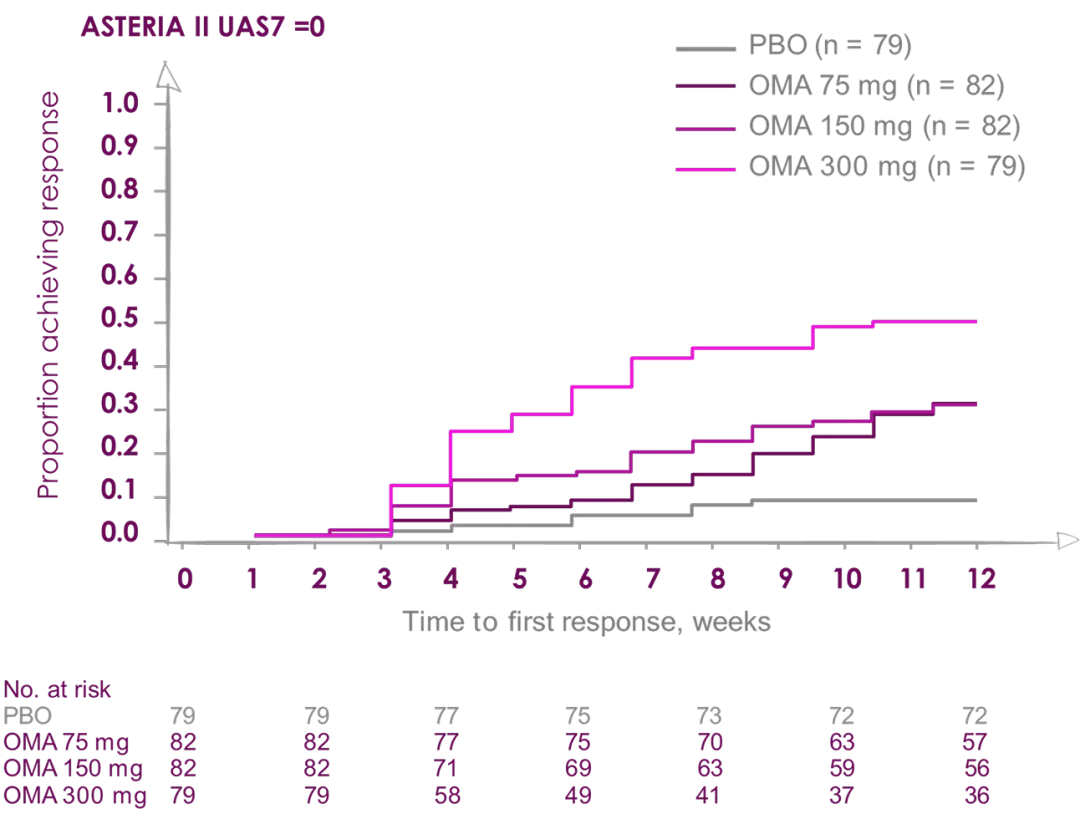
Median time to achieve complete response UAS=0 was 8 weeks in patients receiving Xolair (Omalizumab) 300 mg2
Median time to achieve well-controlled urticaria (UAS7 ≤6) was
6 weeks in patients receiving Xolair (Omalizumab) 300 mg2
Median time to achieve complete response UAS=0 was 12 weeks in patients receiving Xolair (Omalizumab) 300 mg2
Study design:
ASTERIA I and ASTERIA II were phase III, global, randomized, multicenter, doubleblind, placebo-controlled clinical trials designed to assess the efficacy and safety of omalizumab use in patients with CIU/CSU. ASTERIA I and ASTERIA II enrolled patients with CIU/CSU who remained symptomatic despite treatment with H1 antihistamines at approved doses. Enrolled patients in both studies had to have a UAS7 ≥16 (equivalent to moderate-to-severe CIU/CSU symptoms on at least 4 of 7 days) and an itch component UAS7 (range, 0-21) ≥ 8 for the 7 days before randomization.
Enrolled patients were randomized 1:1:1:1 to receive omalizumab (75, 150, or 300 mg) or placebo subcutaneously every 4 weeks for 6 (ASTERIA I) or 3 (ASTERIA II) doses. GLACIAL was a phase III, global, randomized, multicenter, double-blind, placebo-controlled clinical trial that assessed the safety and efficacy of omalizumab use in patients with CIU/CSU. H1-antihistamines had failed at up to 4 times the recommended dose plus H2-antihistamines, LTRAs, or both in GLACIAL-enrolled patients. Enrolled patients were randomized 3:1 to receive 300 mg of omalizumab or placebo subcutaneously every 4 weeks for 6 doses.2
The aim of this analysis was to investigate response patterns of omalizumab to treat CIU/CSU in the phase III clinical trial data, with a goal of providing a practical approach to omalizumab use for clinicians by using omalizumab in the real-world setting.2
Xolair (Omalizumab) 300 mg significantly decreased
Angio-edema quality of life burden at week 24
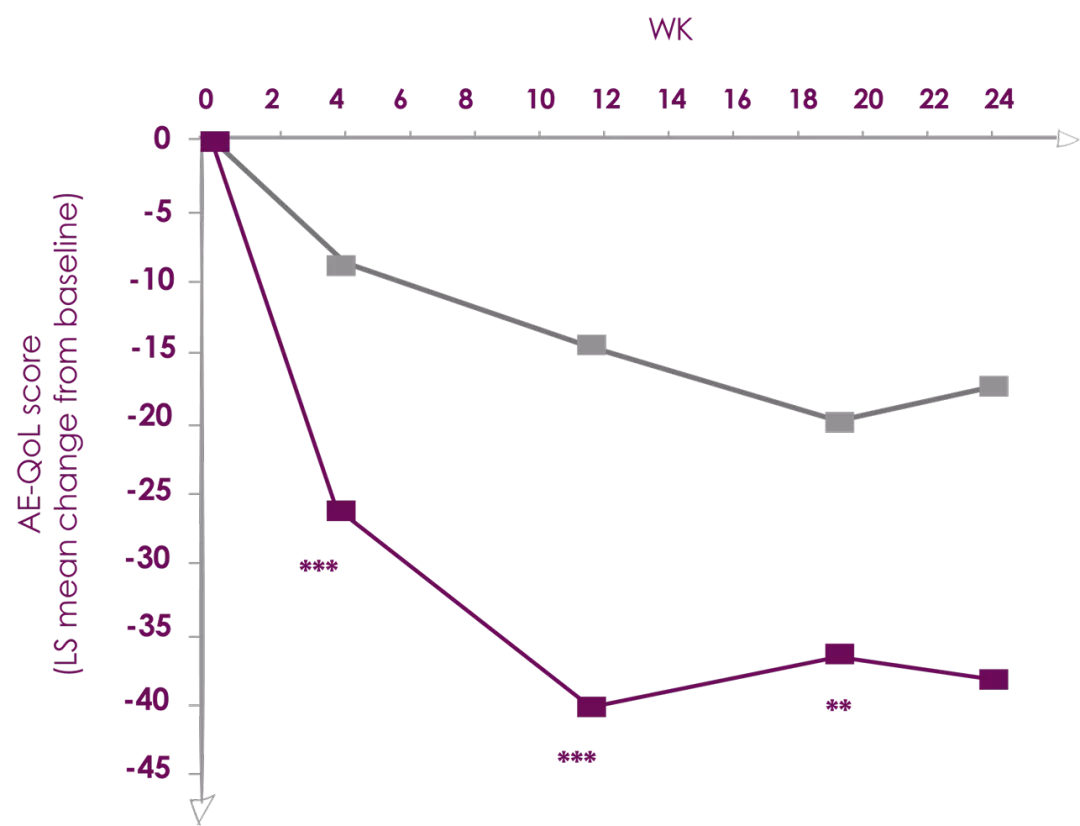
**P<0.01; ***P<0.001. | Adapted from Ref. 3 |
Xolair (Omalizumab) 300 mg significantly decreased Angio-edema score at week 24
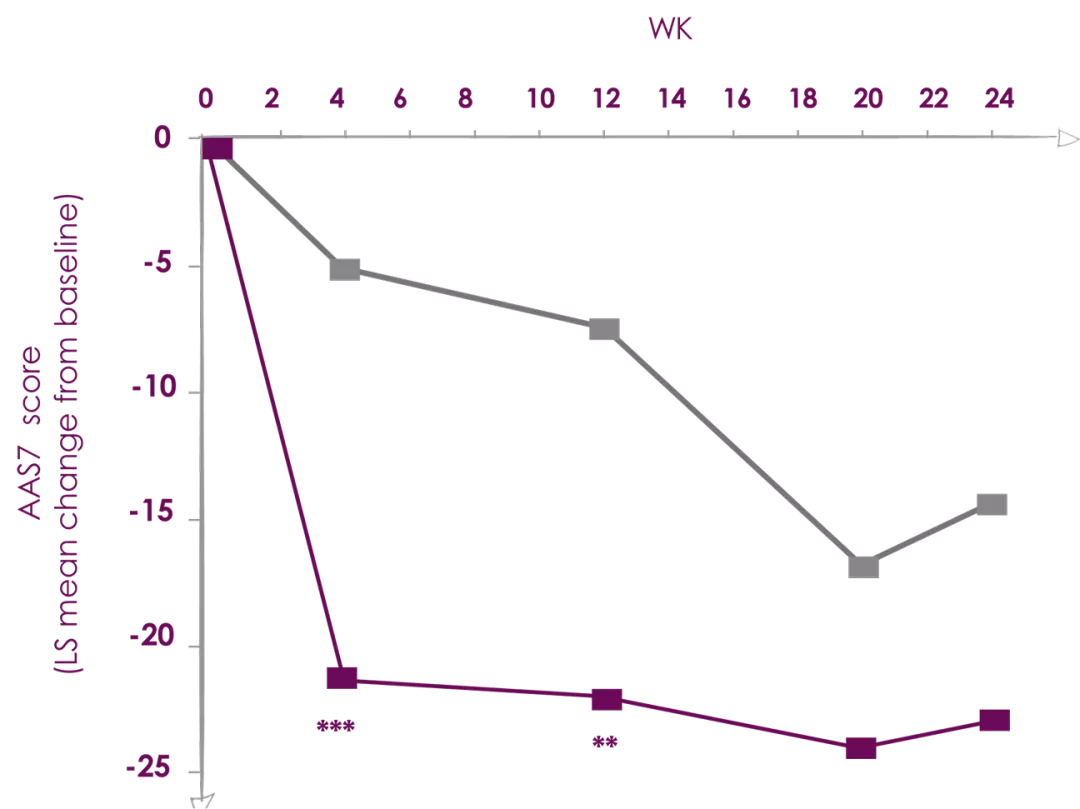
*P<0.05; **P<0.01; ***P<0.001. | Adapted from Ref. 3 |
Study design:3
X-ACT (Xolair Effects on Angioedema in Chronic Spontaneous Urticaria Treatment) was a randomized, double-blind, placebo-controlled, multicenter, phase III study.
The trial was conducted between January 2013 and May 2014 in 24 centers across Germany. Briefly, the study was divided into a 2-week screening period, a 28-week double-blind treatment phase in which patients were randomized (1:1) to receive subcutaneous injections of omalizumab 300 mg or placebo every 4 weeks frombaseline (Week 0) until Week 24, and an 8-week follow up phase. Patients were male and female CSU patients aged 18-75 years with a history of angioedema (at least 4 episodes within the last 6 months before study enrollment), who remain symptomatic despite the use of two-to-four times the recommended dose of second generation H1-antihistamine treatment, with an urticaria activity score (UAS) over 7 days (UAS7) of ≥14 and a CU-Q2oL score of ≥30 at baseline were Included in the study. Number of patients were 35.
Aim is to investigate the effects of omalizumab 300 mg treatment on the impact of angioedema on QoL in patients with moderate-to-severe CSU.
Across Real-world Meta-analysis studies, Xolair (Omalizumab) 300 mg shows4
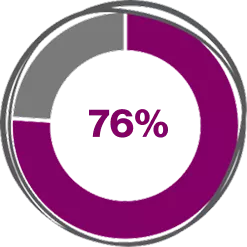
(95%CI, 70.0%-82.0%; z = 27.0, P < .001)
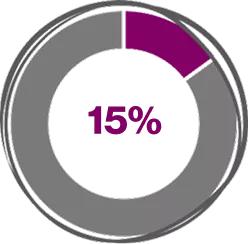
(95% CI, 10.0%-22.0%; z = 7.6, P < .001)
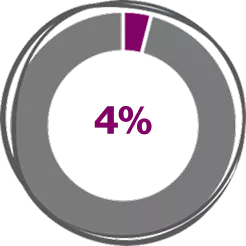
(95%CI, 1.0%-7.0%; z = 3.6, P < .001)
Study design:
Published observational studies and scientific abstracts on the effectiveness of omalizumab in CIU conducted to quantitatively synthesize what is known about the benefits and harms of omalizumab as used in real-world management of CIU using meta-analytic techniques, and to provide insight into potential efficacy-effectiveness gaps. A total of 67 observational studies on the effectiveness of omalizumab in CIU (with or without angioedema)were identified.1 The search was conducted for studies published from January 1, 2006, to January 1, 2018.4
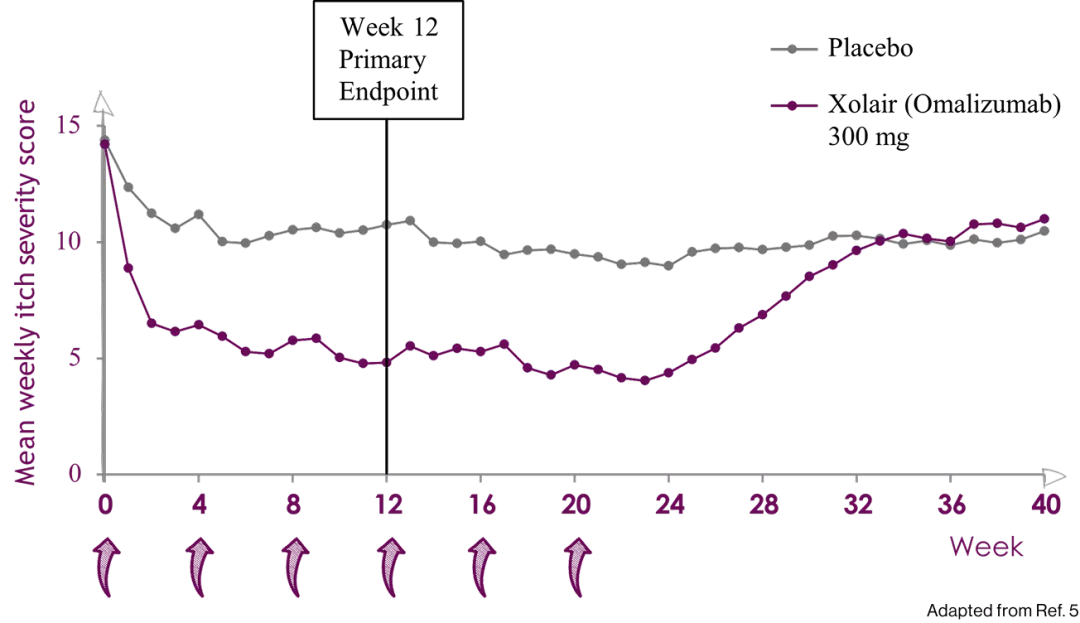
Xolair 300 mg significantly decreased mean weekly itch severity
score over 12 weeks and sustained up to 24 weeks5
Mean weekly itch severity score over time, study 1 (mITT population)
Adverse reactions from the pooled CSU safety database (day 1 to week 24) at 300 mg omalizumab5
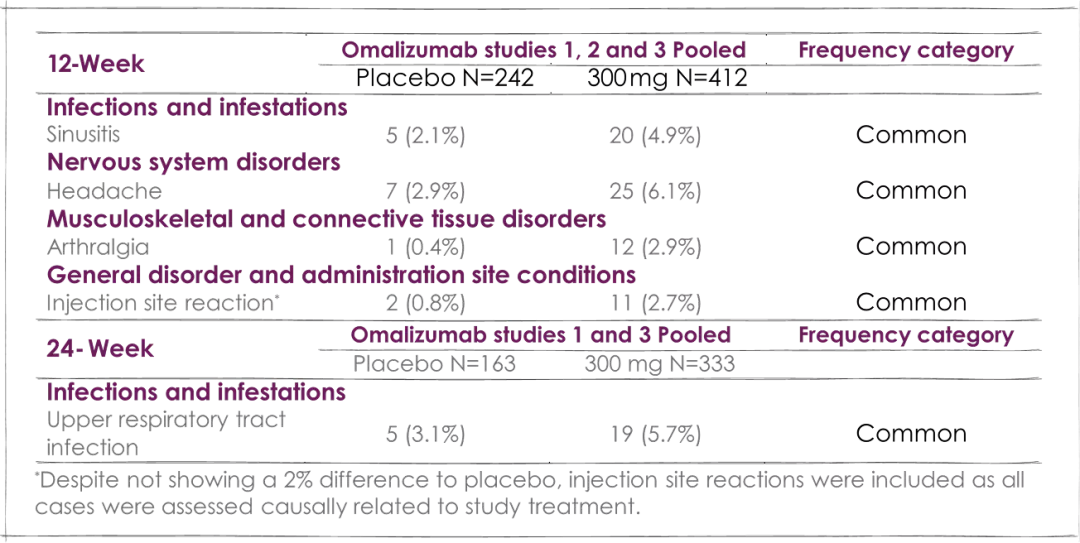
mITT=modified intention-to-treat population: included all patients who were randomised and received at least one dose of study medication.
Xolair API
Xolair API
UAS: Urticaria activity score, PBO: Placebo, OMA: Omalizumab, CIU: Chronic idiopathic urticaria, CSU: Chronic spontaneous urticaria, AE-QoL: Angioedema Quality of Life Questionnaire, AAS: Angioedema Activity Score, API: Abbreviated prescribing information.
References
Xolair® 150 mg powder and solvent for solution for injection Ministry of health (MOH) approved leaflet. approval date: 26/10/2020.
Kaplan A, Ferrer M, Bernstein JA, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. Journal of Allergy and Clinical Immunology. 2016;137(2):474-81.
Staubach P, Metz M, Chapman-Rothe N, et al. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy. 2018;73(3):576-84.
Tharp MD, Bernstein JA, Kavati A, et al. Benefits and harms of omalizumab treatment in adolescent and adult patients with chronic idiopathic (spontaneous) urticaria: a meta-analysis of “real-world” evidence. JAMA dermatology. 2019;155(1):29-38.
Xolair®. Summary of product characteristics (SMPC). Available at: https://www.ema.europa.eu/en/documents/product-information/xolair-epar-product-information_en.pdf. Last accessed at: 08/07/2025.
Approved by Egyptian Drug Authority: HBF0424OA4702/092025. Invalidation date: 03/09/2026.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.
Image

|
HBF0424OA4702/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
