
Kesimpta™
Indication: KESIMPTA™ is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (MS).
All the resources you need to successfully incorporate
KESIMPTA™ in your clinical practice
- The use of premedication is not required1
- Prior to initiating KESIMPTA™, perform hepatitis B virus (HBV) screening, serum immunoglobulins and vaccinate (at least 4 weeks prior for live or live-attenuated vaccines and at least 2 weeks prior for inactivated vaccines)
- The first injection should be performed under the guidance of a health care professional.
How does KESIMPTA™ Work?
In which types of patients with RMS is KESIMPTA™ appropriate?
What is CDW?
What injection training support does Novartis offer?
Were there any cases of hepatitis B observed with KESIMPTA™?
HCP Guides
Getting Started with KESIMPTA™.
When starting KESIMPTA™.
HCP training video
Teach your patients to self-administer KESIMPTA™ with the Sensoready® Pen.
For full instructions how to use
Patients Guides
Self adminitration
KESIMPTA™ offers the simplicity of a self-administered B-cell therapy with the subcutaneous Sensoready® Pen, for a treatment that can be administered at home or anywhere1-4*
The initial dosing period consists of 20 mg subcutaneous doses at Weeks 0, 1, and 2 with once-monthly dosing starting at Week 4.
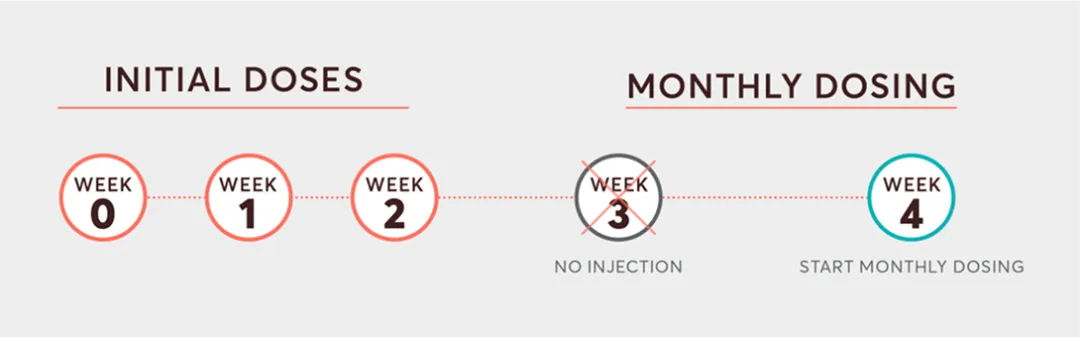
KESIMPTA™ Sensoready® Pens must be refrigerated at 2ºC to 8ºC. Keep product in the original box to protect from light until the time of use. Do not freeze.To avoid foaming, do not shake. KESIMPTA™ does not contain a preservative. The subcutaneous route of administration may offer reduced time in a treatment clinic.3-5
Below you will find materials and services that will assist patients starting KESIMPTA™.
Injection Videos
Teaching patients how to use the Sensoready® Pen
Dosing and monitoring
Dosing Regimen with KESIMPTA™
After initial dosing, KESIMPTA™ is administered once monthly1
KESIMPTA™ Dosing regimen
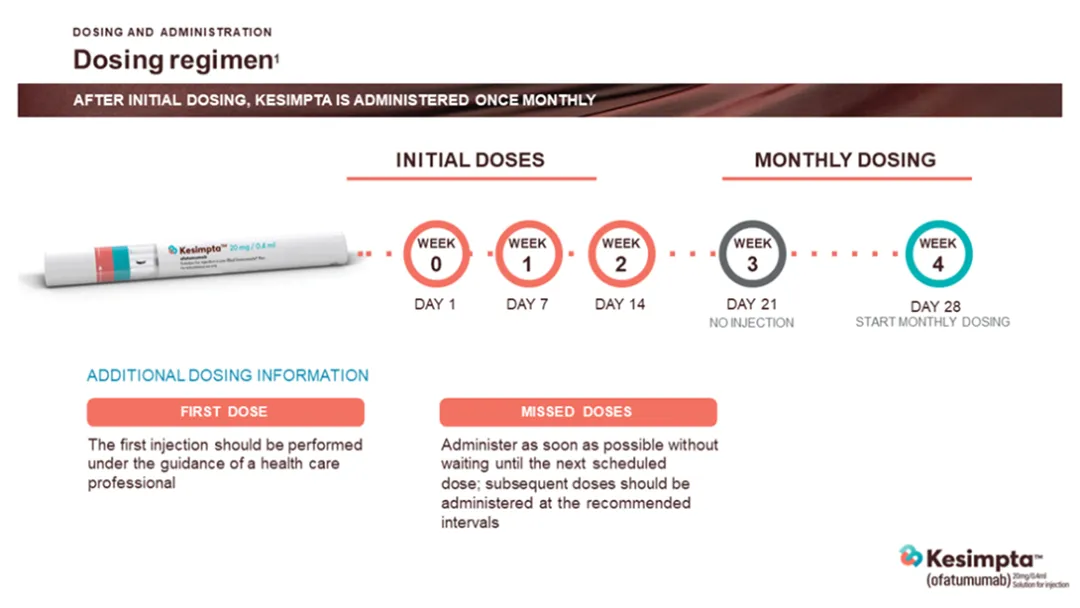
Additional dosing information:
First dose: KESIMPTA™ should be initiated by a physician experienced in the management of neurological conditions. The first injection of KESIMPTA™ should be performed under the guidance of a healthcare professional.1
Missed doses: Administer as soon as possible without waiting until the next scheduled dose.
Subsequent doses should be administered at the recommended intervals (e.g. 1 month intervals).
FAQ'S
The precise mechanism by which ofatumumab exerts its therapeutic effects in multiple sclerosis is unknown, but is presumed to involve binding to CD20, a cell surface antigen present on pre-B and mature B lymphocytes. Following cell surface binding to B lymphocytes, ofatumumab results in antibody-dependent cellular cytolysis and complement-mediated lysis.1
KESIMPTA™ is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (RMS)
KESIMPTA™ was studied in a broad range of patients in two Phase 3 clinical trials.
>> Approximately 40% of patients in KESIMPTA™ clinical studies were DMT-treatment naïve
>> The study population included patients 18-55 years of age with an EDSS score of 0 to 5.5.1,2
A key clinical outcome measured in the pivotal studies was confirmed disability worsening (CDW).
Disability worsening at 3 months or 6 months was defined as an increase from baseline in the Expanded Disability Status Scale (EDSS) For patients with a baseline EDSS score of 0, an increase in the EDSS score .5 points was required. For patients with a baseline EDSS score of 1.0 to 5.0, the criterion was an increase 1.0 point; and for patients with a baseline EDSS score of at least 5.5 points, the criterion was an increase 0.5 points.2
The first injection of KESIMPTA™ should be performed under the guidance of a healthcare professional.
KESIMPTA™ also offers in-home injection training for the first injection with the below supplemental resources:
No cases of hepatitis B virus (HBV) reactivation were identified in KESIMPTA™ clinical studies.
Hepatitis B reactivation has occurred in patients treated with anti-CD20 antibodies, which in some cases resulted in fulminant hepatitis, hepatic failure, and death. Patients with active hepatitis B disease should not be treated with KESIMPTA™.
All patients should be screened for hepatitis B before starting KESIMPTA™
CDW, confirmed disability worsening; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; RMS, relapsing multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
KESIMPTA™ API
KESIMPTA™ API
References
Kesimpta insert leaflet, Novartis Pharma AG, Switzerland.
Hauser SL, Bar-Or A, Cohen JA, et al, for the ASCLEPIOS I and ASCLEPIOS II trial groups. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546-557.
De Cock E, Kritikou P, Sandoval M, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One. 2016;11(6): e0157957. doi:10.1371/journal.pone.0157957.
Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol. 2017;28(4):836-842.
Dieguez G, Engel T, Jacobson N; for Novartis Pharmaceuticals Corporation. Site of service and cost dispersion of infused drugs: a case study of patients with multiple sclerosis.Milliman white paper. Novartis Pharmaceuticals Corp; East Hanover, NJ. December 2019.
