
Kesimpta™
Indication: KESIMPTA™ is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (MS).
- Primary endpoint was ARR1
- Secondary endpoints included number of Gd+ T1 lesions, rate of new or enlarging T2 lesions, and 3- and 6-month CDW1
- Primary endpoint: ARR
- Secondary MRI endpoints: number of Gd+T1 lesions per scan; annualised rate of new or enlarging T2 lesions
- Secondary clinical endpoint: 3-month and 6-month CDW
Novartis Pharma AG, Switzerland.
Gupta IV, Jewell RC. Ofatumumab, the first human anti-CD20 monoclonal antibody for thetreatment of B cell hematologic malignancies. Ann NY Acad Sci. 2012;1263(1):43-56.
Huck C, Leppert D, Wegert V, et al. Low-dose subcutaneous anti-CD20 treatment depletes disease relevant B cell subsets and attenuates neuroinflammation. J Neuroimmune Pharmacol. 2019;14(4):709-719.
Torres JB, Roodselaar J, Sealey M, et al. Distribution and efficacy of ofatumumab and ocrelizumab in humanized-CD20 mice following subcutaneous or intravenous administration. Poster P2.2.052.Presented at: American Academy of Neurology Annual Meeting; May 4-10, 2019; Philadelphia, PA.
Theil D, Smith P, Huck C, et al. Imaging mass cytometry and single-cell genomics reveal differential depletion and repletion of B-cell populations following ofatumumab treatment in cynomolgus monkeys. Front lmmunol. 2019;10:1-11.
Hauser SL, Bar-Or A, Cohen JA, et al. B-cell depletion and efficacy outcomes with ofatumumab: subgroup analysis from the pooled phase 3 ASCLEPIOS I and II trials. Poster P7.1-013. Presented at: American Academy of Neurology Annual Meeting; May 4-10, 2019; Philadelphia, PA.
Hauser SL, Bar-Or A, Cohen JA, et al; for the ASCLEPIOS I and ASCLEPIOS II trial groups. Ofatumumab versus teriflunomide in multiple sclerosis [supplemental appendix]. N Engl J Med. 2020;383(6):546-557.
- In the Phase 3 pivotal clinical studies, 51.6% of KESIMPTATM -treated patients experienced at least one infection vs 52.7% of teriflunomide-treated patients.
- Injection-related reactions (systemic) and injection-site reactions (local) were reported in 21% and 11% of patients treated with KESIMPTATM , compared to 15% and 6% in the teriflunomide treated patients respectively.
- The incidence of injection-related reactions (systemic) was highest with the first injection (14.4%), decreasing significantly with subsequent injections (4.4% with second, <3% from third injection)
- Injection-related reactions were mostly (99.8%) mild to moderate in severity. Two (0.2%) KESIMPTATM -treated RMS patients reported serious injection-related reactions.
- Pooled data from both clinical trials show that treatment discontinuation rates due to adverse events were similar between KESIMPTATM (5.7%) and teriflunomide (5.2%)1,3
- The most common cause of discontinuation in patients treated with KESIMPTATM was low IgM (3.3%), defined in trial protocols as IgM at 10% below the LLN1
- Hypersensitivity to the active substance or any of the excipients
- Severely immunocompromised patients
- Presence of an active infection
- Known active malignancies.
- Treatment initiation during pregnancy
ASCLEPIOS Trial: Efficacy
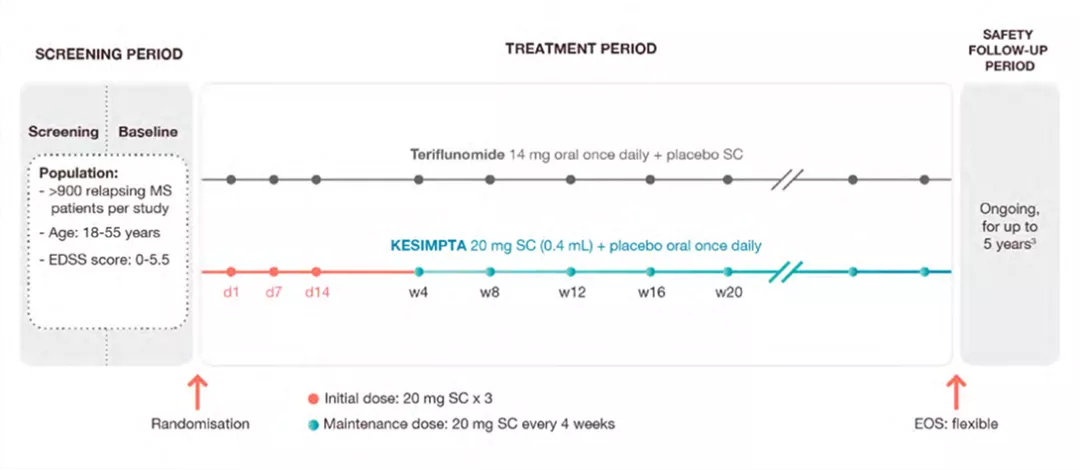
ASCLEPIOS I & II Study Design
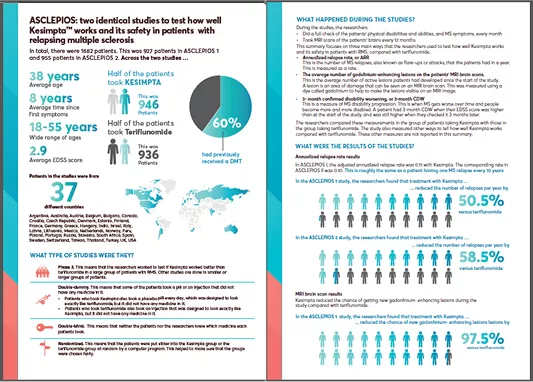
ASCLEPIOS I and II were 2 identical, double-blind, active comparator-controlled, parallel-group, multicenter, Phase 3 studies in patients with RMS (N=1882), approximately 40% of whom were DMT treatment naïve.1
Patients were randomized to double-dummy subcutaneous KESIMPTA™ (20 mg subcutaneously every 4 weeks after 20mg loading doses at days 1, 7 and 14) or oral teriflunomide (14 mg once daily) for up to 30 months.1
Primary endpoint was ARR
Primary endpoint
KESIMPTA™ demonstrated a significant reduction in relapses of up to nearly 60% vs teriflunomide (P<0.001) *1,2
Relative reduction in annualised relapses vs teriflunomide1,2
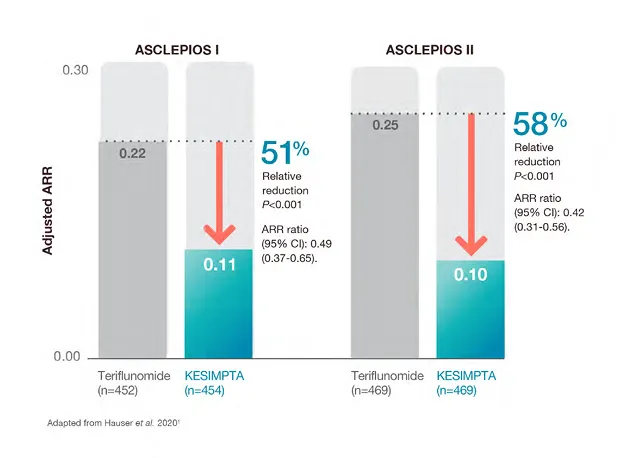
*Based on ARR primary endpoint results, 0.1 relapses per year = 1 relapse every 10 patient-years.1,2
MRI Endpoints
Secondary endpoint
ASCLEPIOS included 2 key MRI endpoints: number of Gd+ T1 lesions and new or enlarging T2 lesions. KESIMPTA™ demonstrated improvements in both endpoints vs teriflunomide.1,2
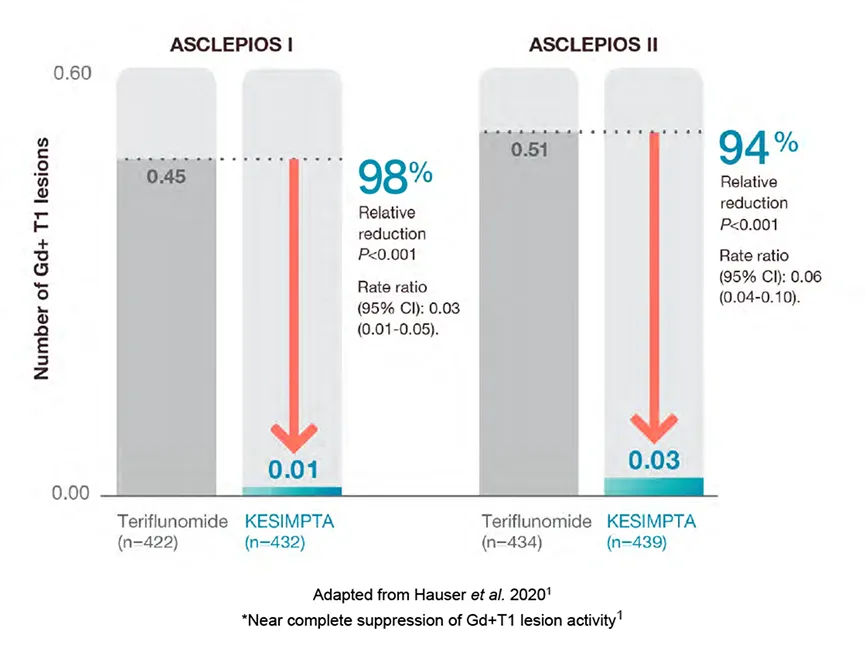
1. Number of Gd+ T1 lesions
KESIMPTA™ treatment resulted in a significant reduction in GD+ T1 lesions vs teriflunomide (P<0.001)*1,2
Mean number of Gd+T1 lesions per scan1,2
2. T2 lesions
KESIMPTA™ treatment resulted in superior reduction in T2 lesions vs teriflunomide (P<0.001)*1,2
Mean number of new or enlarging T2 lesions per year1,2
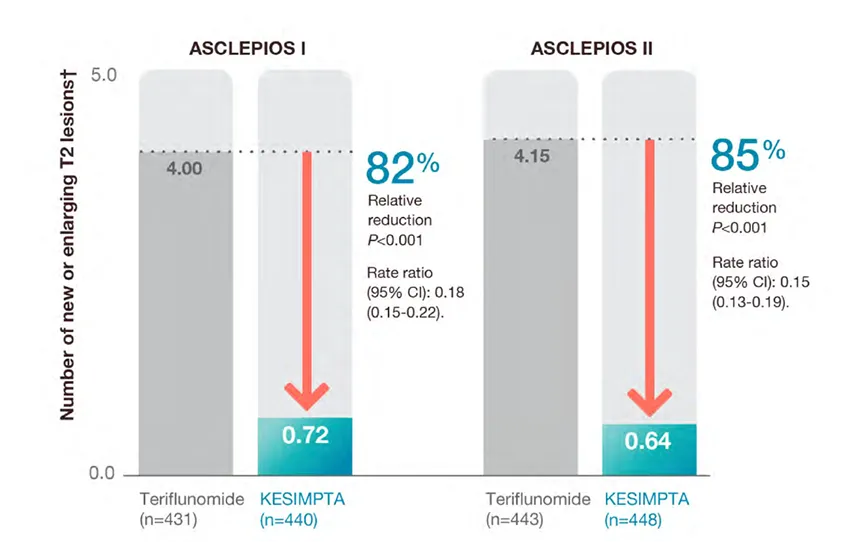
Adapted from Hauser et al. 20201
*Near complete suppression of Gd+T1 lesion activity1
Confirmed Disability Worsening (CDW)
Confirmed Disability Worsening
KESIMPTA™ treatment resulted in a significant reduction in relative risk of confirmed disability worsening (CDW) vs teriflunomide (P=0.002)*1,2
In a prespecified meta-analysis of pooled data from ASCLEPIOS I and II.
CDW in ASCLEPIOS I and II
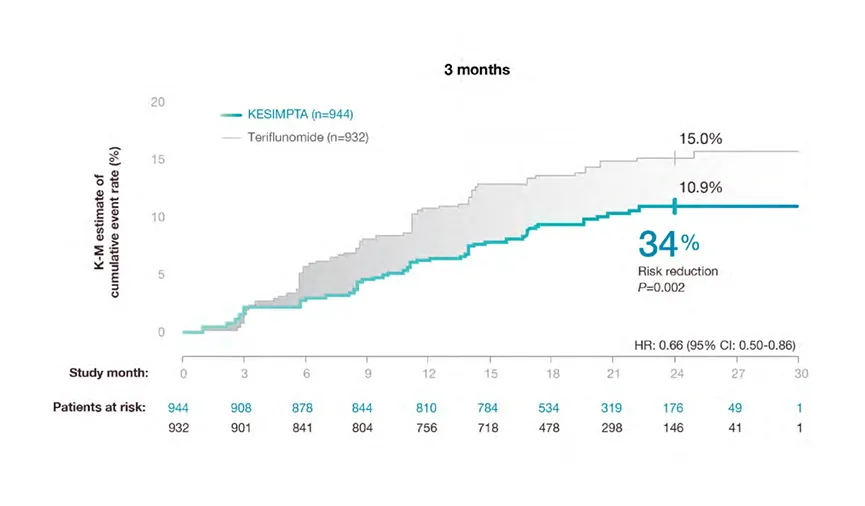
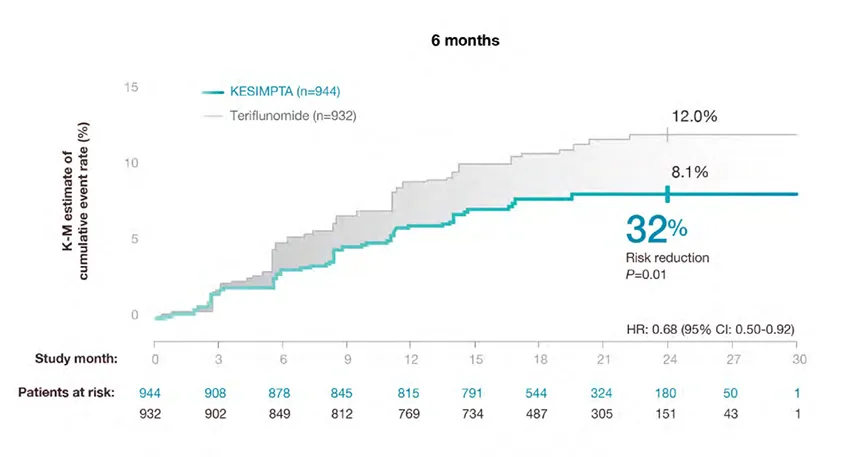
*Disability worsening confirmed at 3 months or 6 months was defined as an increase from baseline in the Expanded Disability Status Scale
(EDSS) score (on a scale from 0 to 10.0, with higher scores indicating worse disability) that was sustained for at least 3 or 6 months. For patients
with a baseline EDSS score of 0, an increase in the EDSS score of at least 1.5 points was required; for patients with a baseline EDSS score of 1.0
to 5.0, the criterion was an increase of at least 1.0 point; and for patients with a baseline EDSS score of at least 5.5 points, the criterion was an
increase of at least 0.5 points2.
NEDA-3
Post-Hoc: NEDA-3 (No Evidence of Disease Activity)
NEDA-3 is a combination of three measures related to MS disease activity: (a) no relapses; (b) no new MRI activity6 and (c) no disability progression measured by EDSS7,8.
These are reflected in:
NEDA-3* post hoc analysis4
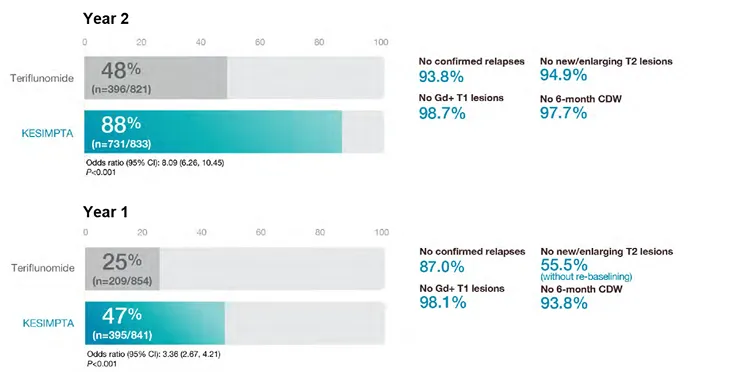
Nearly 9 out of 10 patients taking KESIMPTA™ achieved NEDA-3 in Year 2
No conclusions on clinical outcomes can be drawn.
NEDA-3 Post Hoc Analysis Design
KESIMPTATM Mechanism of Action
Targeted B-Cell Therapy
Cell Surface: KESIMPTATM provides a targeted B-cell therapy.1-5
KESIMPTATM is thought to work by Selectively binding to sites of CD-20 on mature B-cells.*2
Cell View - Lysis of B-cells when KESIMPTA™ binds to CD-201
KESIMPTATM causes CD20+ B-cells to be vulnerable to both immediate and delayed B-cell lysis by mechanisms such as complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC).*2
Organ view - KESIMPTA™ promotes specific targeting of the lymph nodes following subcutaneous admin.
Subcutaneous administration of KESIMPTA™ is thought to promote preferential depletion of B-cells in the lymph nodes. Preclinical evidence suggests KESIMPTA™ may preserve B-cells in the spleen that help maintain immune function.*4,5
B-Cell Depletion
KESIMPTA™ is a subcutaneous, targeted, and precisely delivered therapy that provides rapid B-cell depletion6,7
B-cell depletion sustained over the dosing period
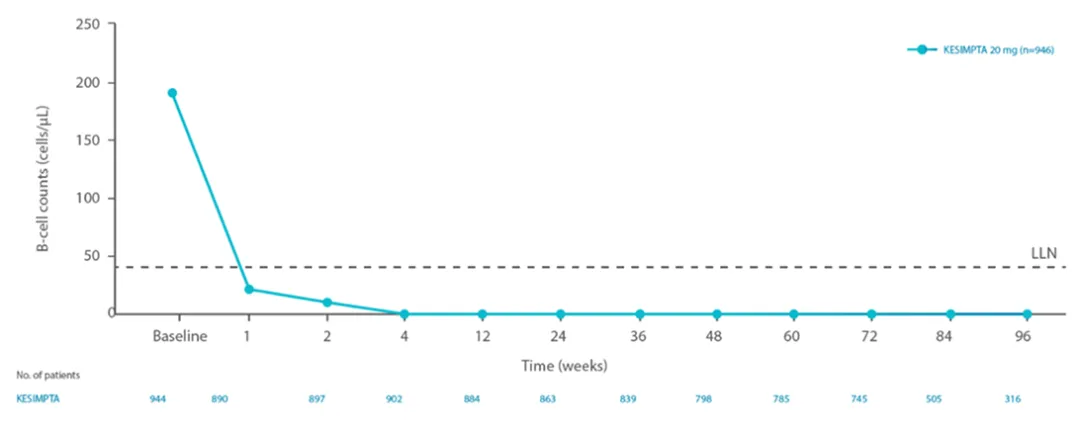
Reduction in B-cells was seen as early as 1 week after treatment initiation1
The CD19+ B-cell counts remained below LLN for approximately 97% of patients in ASCLEPIOS I and 92% of patients in ASCLEPIOS II from 12 weeks through 120 weeks while on KESIMPTA™ treatment.1
B-Cell Repletion Upon Discontinuation1
Data from RMS clinical studies indicate B-cell recoveries over the LLN in at least 50% of patients in 24-36 weeks post treatment discontinuation1
Modelling and simulation for B-cell repletion corroborates these data, predicting median time to B-cell recovery of 40 weeks post treatment discontinuation1
Mechanism of Action video
LLN, lower limit of normal; RMS , relapsing multiple sclerosis.
References
KESIMPTA™ Safety
Adverse Events (AEs)
KESIMPTA™ safety was assessed across pooled data from ASCLEPIOS I and II studies1
Adverse events in patients with RMS with an incidence of at least 5% with KESIMPTA™ and a greater incidence than teriflunomide.
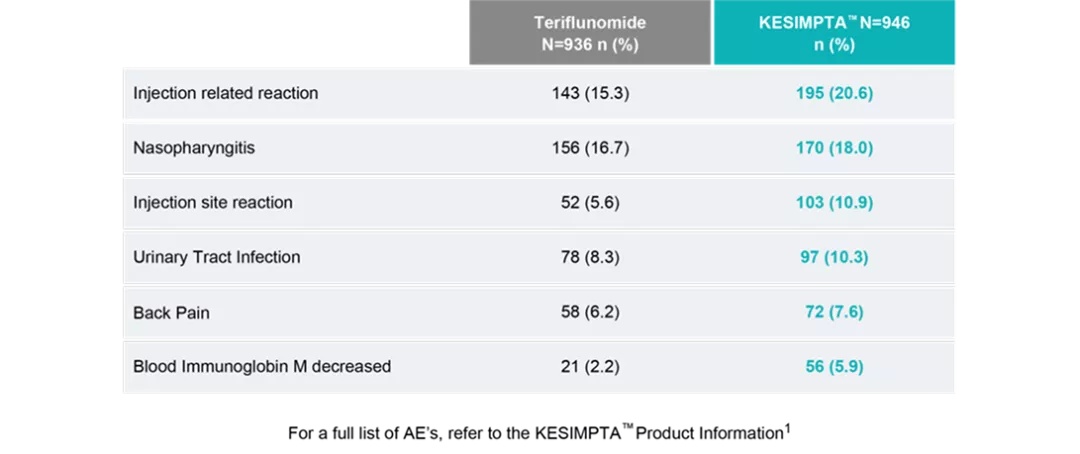
In clinical trials, systemic injection reactions with KESIMPTA™ occurred most commonly within 24 hours of the first injection but were also observed with later injections.
The overall rate of infections and serious infections in patients treated with KESIMPTA™ was similar to patients who were treated with teriflunomide (51.6% vs 52.7%, and 2.5% vs 1.8%, respectively).1
Tolerability Profile
Safety across pooled ASCLEPIOS I and II studies1,3
Treatment Discontinuations
Pooled data from ASCLEPIOS I and II studies
Important Safety Information
Indication
KESIMPTA™ is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (MS).
Important Safety Information
Contraindication:
Warnings and Precautions
For a full list of warnings and precautions, please refer to the KESIMPTA™ Prescribing Information.
ASCLEPIOS Publication Explainer
ARR, annualised relapse rate; CDW, confirmed disability worsening; CI, confidence interval; EDSS, Expanded Disability Status Scale; Gd+, gadolinium-enhancing; HR, hazard ratio; K-M, Kaplan-Meier; MRI, magnetic resonance imaging; NEDA, No evidence of disease activity; RMS, relapsing multiple sclerosis.
KESIMPTA™ API
KESIMPTA™ API
References
Kesimpta insert leaflet, Novartis Pharma AG, Switzerland.
Hauser SL, Bar-Or A, Cohen JA, et al. for the ASCLEPIOS I and ASCLEPIOS II trial groups. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546-557.
Hauser S, Bar-Or A, Cohen J, et al. Ofatumumab versus teriflunomide in relapsing multiple sclerosis: analysis of no evidence of disease activity (NEDA-3) from ASCLEPIOS I and II trials. Eur J Neurol. 2020;27(S1):85-86.
Hauser SL, Bar-Or A, Cohen JA, et al. B-cell depletion and efficacy outcomes with ofatumumab: subgroup analysis from the pooled phase 3 ASCLEPIOS I and II trials. Poster P7.1-013. Presented at: American Academy of Neurology Annual Meeting; May 4-10, 2019; Philadelphia, PA.
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015 Jul;4(4):329-33.
Banwell B, Giovannoni G, Hawkes C, Lublin F. Editors' welcome and a working definition for a multiple sclerosis cure. Mult Scler Relat Disord. 2013 Apr;2(2):65-7.
Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, Hamlett A, Viglietta V, Greenberg S; CLARITY study group. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011 Apr;10(4):329-37.
Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, Polman CH, O'Connor PW, Giovannoni G, Phillips JT, Lublin FD, Pace A, Kim R, Hyde R. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM)
