
ENTRESTO®
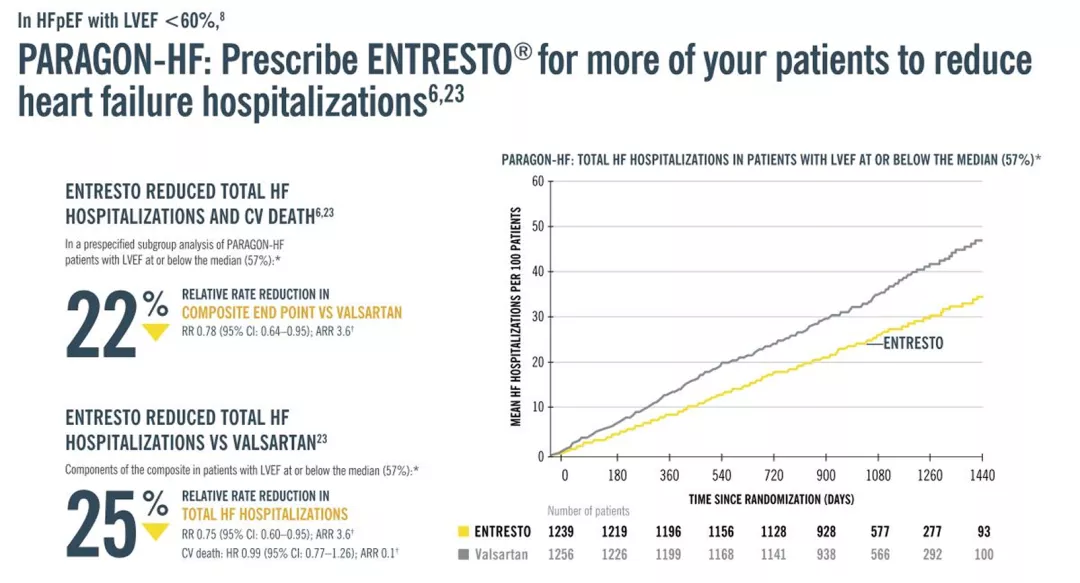
is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction (LVEF) below normal.6
LVEF is a variable measure, so use clinical judgment in deciding whom to treat.
Abbreviations
*LVEF is a variable measure that can change over time, and the normal range differs according to patient characteristics and method of assessment.
† Event rate per 100 patient-years.
ARR, absolute rate reduction; RR, rate ratio; RRR, relative rate reduction.
ENTRESTO® NSS - UAE
ENTRESTO® NSS - UAE
References
ENTRESTO [prescribing information]. Novartis Pharmaceutical Corporation East Hanover, NJ; April 2024
Vaduganathan M, Claggett BL, Greene SJ, et al.Potential implications of expanded US food and drug administration labeling for sacubitril/valsartan in the US. JAMA Cardiol. Published online September 15, 2021. DOI:10.1001/jamacardio.2021.3651
Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609-1620. DOI:10.1056/ NEJMoa1908655
US Food and Drug Administration. Novartis cardiovascular and renal drugs advisory committee briefing document, December 15, 2020. ENTRESTO® (sacubitril/valsartan) for chronic heart failure and preserved ejection fraction. Accessed February 7, 2023. https://www.fda.gov/media/144379/download
Mentz RJ, Ward JH, Hernandez AF, et al. Angiotensin-neprilysin inhibition in patients with mildly reduced or preserved ejection fraction and worsening heart failure. J Am Coll Cardiol. 2023;S0735-1097(23):05429-3. doi:10.1016/j.jacc.2023.04.019.
Data on file. Study CLCZ696DUS01. Novartis Pharmaceuticals Corp; 2023. To be provided if requested
Mentz RJ, Ward JH, Hernandez AF, et al. Angiotensin-neprilysin inhibition in patients with mildly reduced or preserved ejection fraction and worsening heart failure. J Am Coll Cardiol. 2023;S0735-1097(suppl):05429-3. doi:10.1016/j.jacc.2023.04.019.
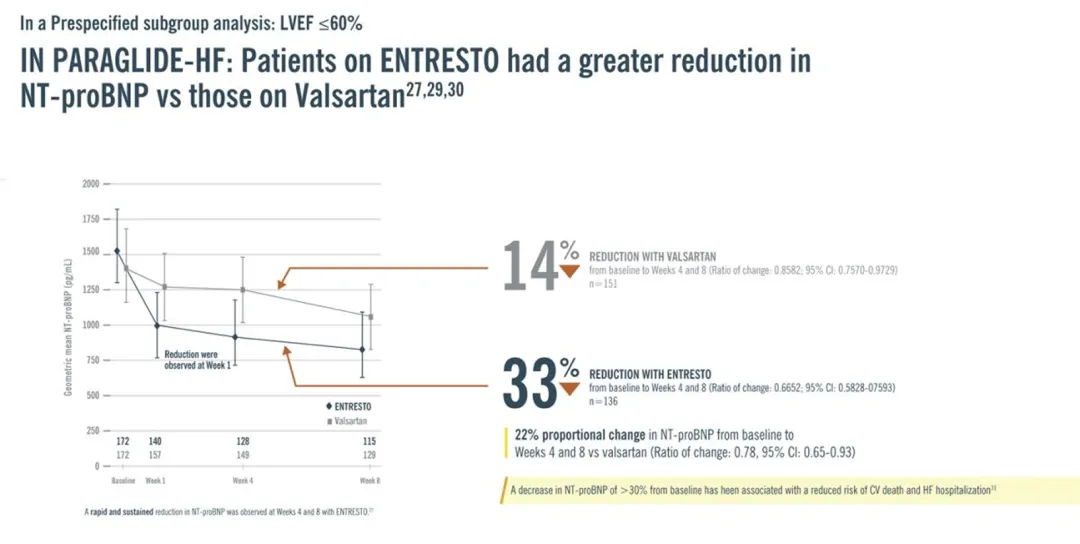
Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168-2174. doi:10.1161/01.CIR.0000144310.04433.BE.
Mentz RJ, Ward JH, Hernandez AF, et al. Rationale, design and baseline characteristics of the PARAGLIDE-HF trial: sacubitril/valsartan vs valsartan in HFmrEF and HFpEF with a worsening heart failure event [published online ahead of print, February 14, 2023]. J Card Fail. 2023;S1071-9164(23)00040-4. doi:10.1016/j.cardfail.2023.02.001
