
ORION-8: Long-term assessment of the efficacy and safety of inclisiran in patients with high risk of cardiovascular events
Study Design1
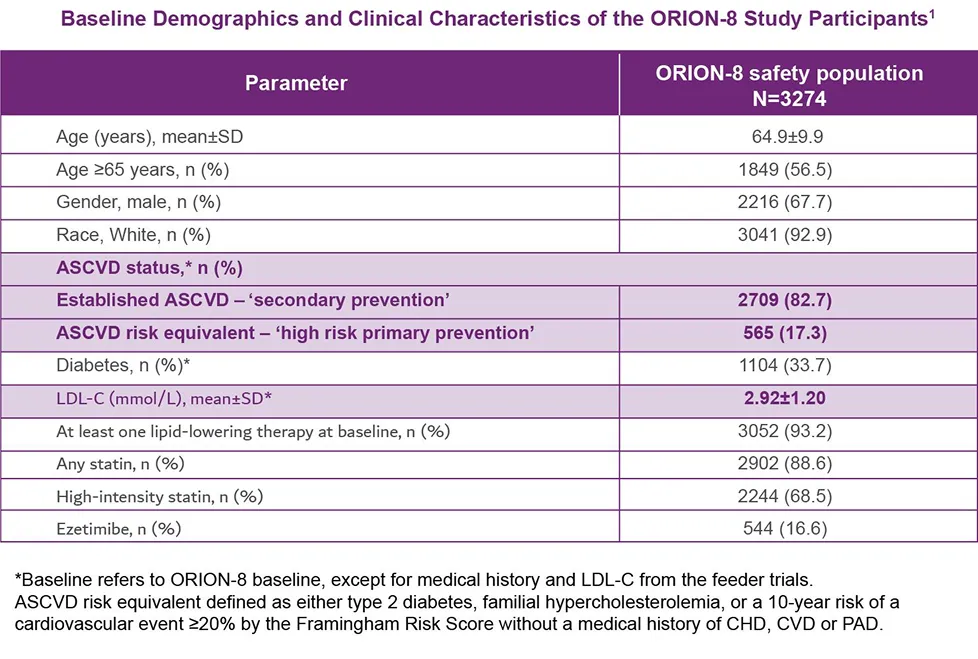
ORION-8 is an open-label extension study with up to 3 years of follow-up in patients who completed pivotal and phase II trials.
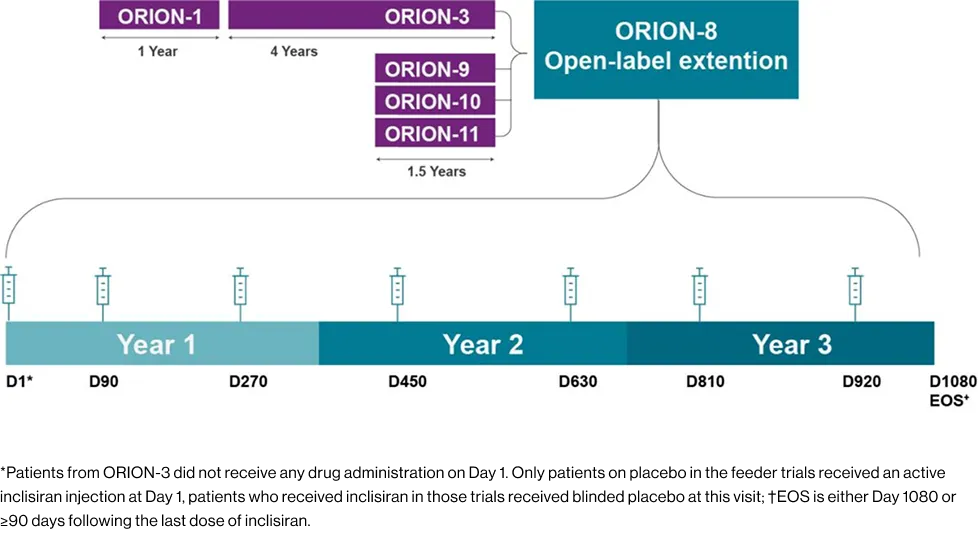
Primary end point1:
The proportion of patients achieving pre-specified LDL-C goals at EOS*
Safety
Secondary endpoint1:
Percent change in LDL-C from baseline to EOS
EOS is either Day 1080 or ≥90 days following the last dose of inclisiran.
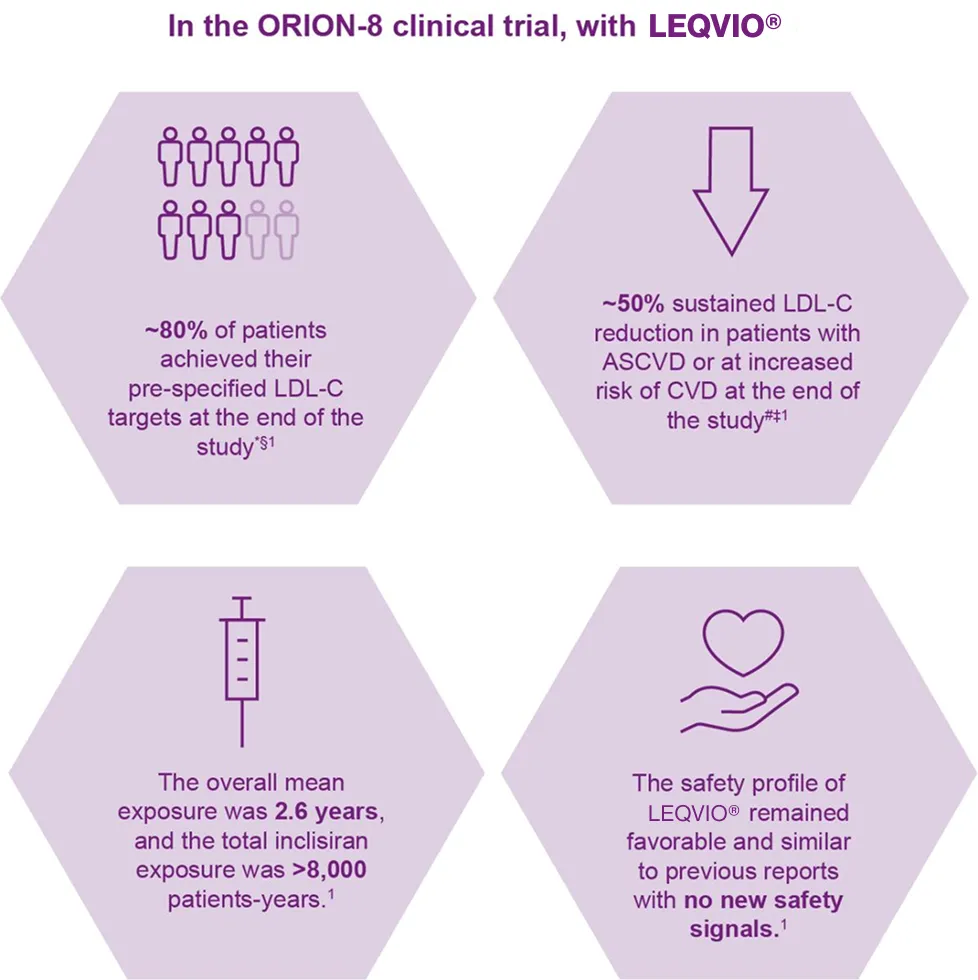
With a cumulative treatment duration of up to 6.8 years, ORION-8 demonstrated that twice-yearly administration of LEQVIO® provides consistent and effective LDL-C-lowering and is well tolerated in patients with high cardiovascular risk, with no new safety signals identified.1
With LEQVIO® treatment most patients achieved their pre-specified lipid goals1
Pre-specified lipid goals: ASCVD <1.8 mmol/L (<70 mg/dL); ASCVD risk equivalent <2.6 mmol/L(<100 mg/dL)1
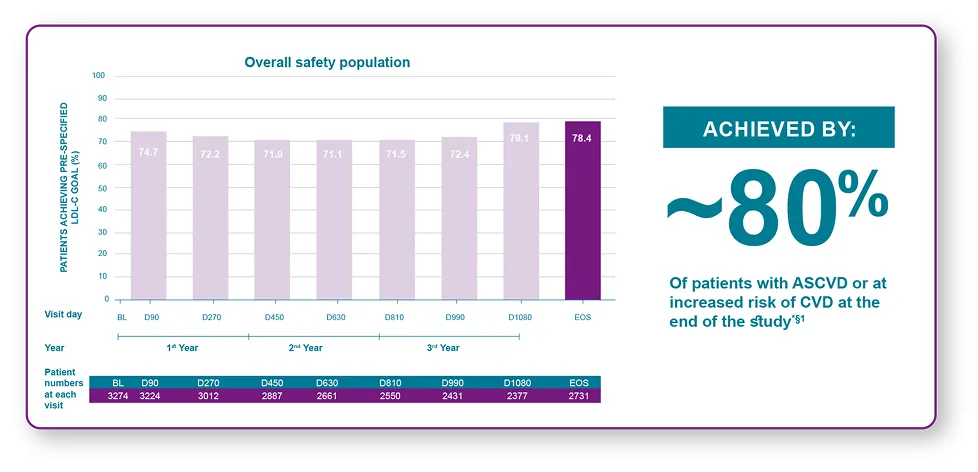
*(78%; 95% CI: 77, 80). §LDL-C target was <70 mg/dL for patients with ASCVD and <100 mg/dL for patients at increased risk for CVD and aligns with AHA/ACC guidelines.
LEQVIO® demonstrated effective and sustained reduction of LDL-C1
LEQVIO® EFFICACY WAS CONSISTENT WITH THE PHASE 3 TRIALS ORION-9, -10 AND -111
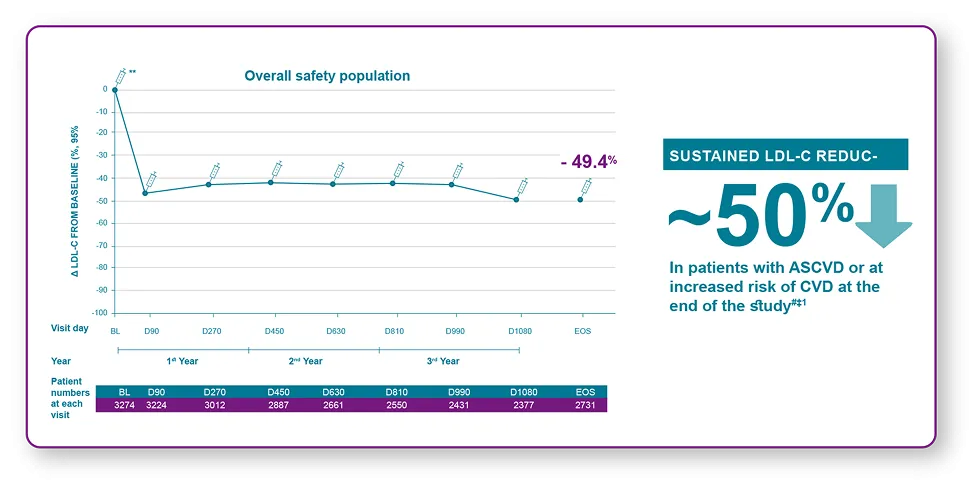
**Baseline value of LDL-C is taken from the baseline of feeder trials. #n=2731 (49%; 95% CI: 48%, 50%). ‡Factors that increase risk of CVD include HeFH, T2DM, or 10-year risk of ≥20%.
Overall exposure to LEQVIO® was 2.6 years, and the total exposure was >8,000 patients-years1,2
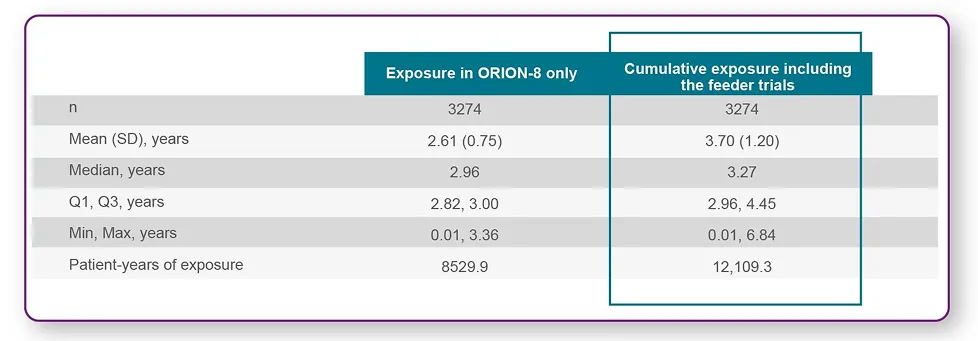
• The cumulative exposure of LEQVIO® was over 12,000 patient-years. The longest exposure was 6.84 years, and a quarter of patients were exposed to LEQVIO® for over 4.45 years.1,2
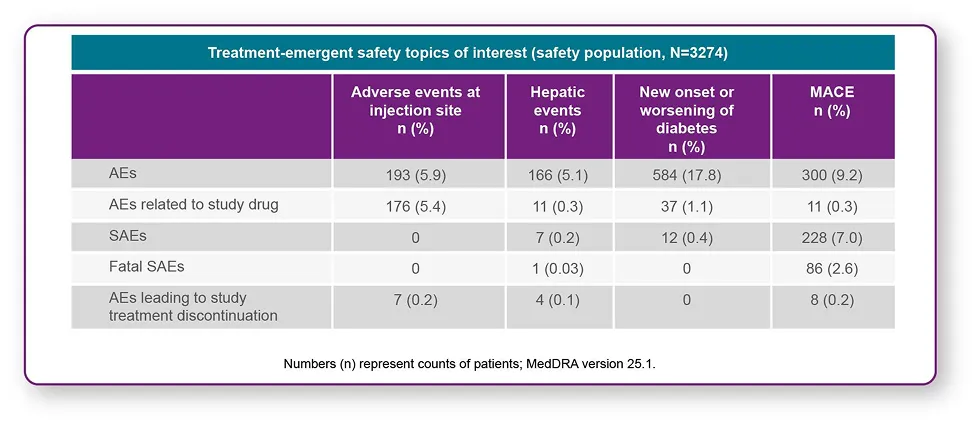
Long-term safety data were consistent with phase III trials1,3
- No new safety signals
- Injection site reactions were the most common causes for treatment discontinuation (0.2% patients taking LEQVIO®)
- LEQVIO®-associated ADAs were infrequent (5.1%), and did not impact the efficacy and safety of LEQVIO®.
Abbreviations:
ASCVD, atherosclerotic cardiovascular disease; CHD, congenital heart disease; CVD, cardiovascular disease; LDL-C, low-density lipoprotein cholesterol; N, total number of patients; n, number of patients in each category; PAD, peripheral artery disease; SD, standard deviation; D, day; EOS, end of study; ACC, American College of Cardiology; AHA, the American Heart Association; HeFH, Heterozygous familial hypercholesterolemia; T2DM, Type 2 diabetes mellitus; AE, adverse event; MACE, major cardiac adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event; TEAE, treatment-emergent adverse event; ADA, anti-drug antibody; Min, minimum; Max, maximum; Q, quartile.
LEQVIO® NSS - UAE
LEQVIO® NSS - UAE
References
Wright RS, Raal FJ, Koenig W, et al. Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: the ORION-8 trial. Cardiovasc Res. 2024 May 16:cvae109. doi: 10.1093/cvr/cvae109.
Wright RS, Raal FJ, Koenig W, et al. Inclisiran administration potently and durably lowers LDL-C over an extended-term follow-up: the ORION-8 trial. Cardiovasc Res. 2024 May 16:cvae109. Supplementary appendix.
LEQVIO® Summary of product characteristics. Novartis Europe Limited.
