
Safety profile
A well-established safety profile with KISQALI® + ET1-3
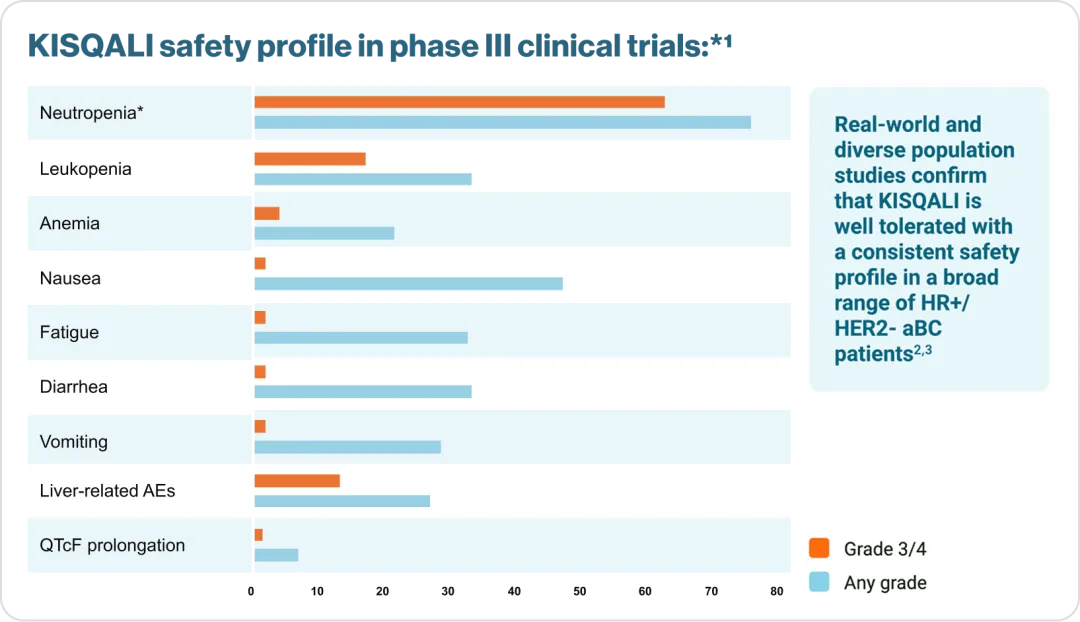
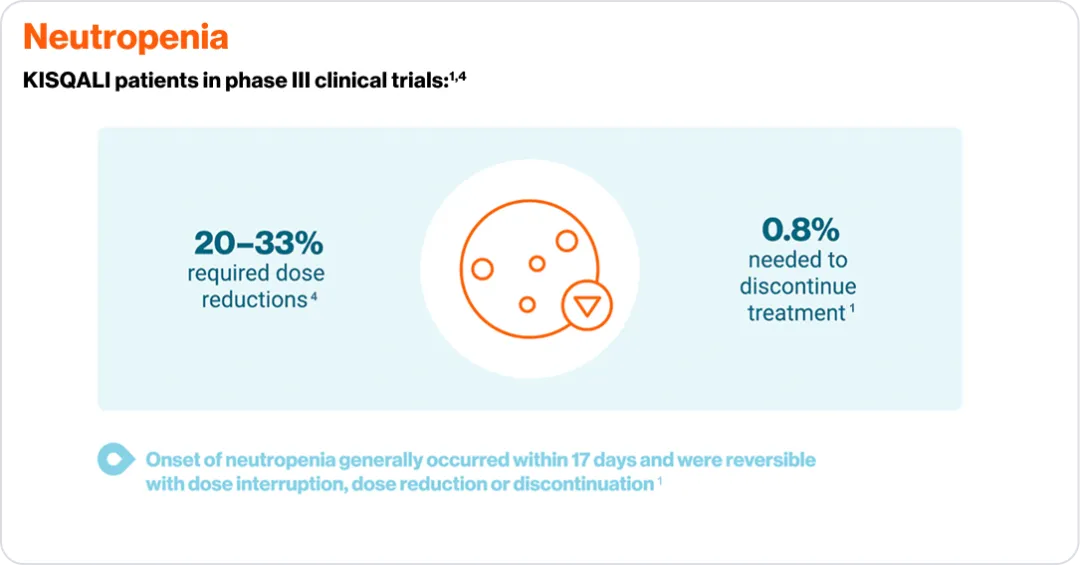
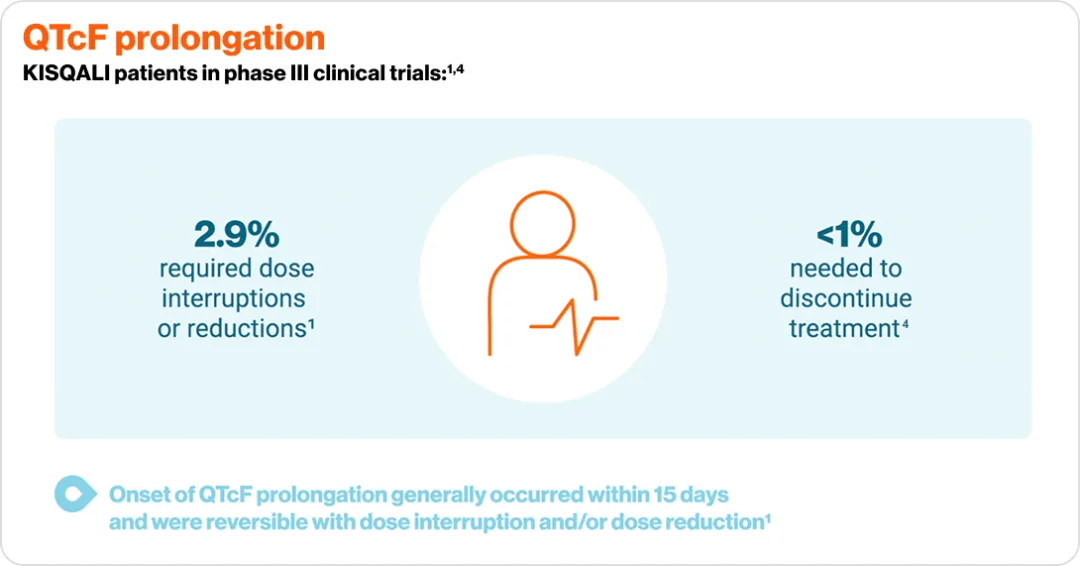

*Please consult your local KISQALI (ribociclib). Prescribing Information for the full KISQALI® safety and tolerability profile.
†Febrile neutropenia was reported in 1.7% of patients exposed to KISQALI® in the phase III clinical studies.1
**To normalisation or grade ≤2.
MONALEESA-2: N=668, double-blind, placebo-controlled, 1:1 randomised, multicentre, phase III trial in postmenopausal women with HR+/HER2− aBC. As 1L in advanced disease. No prior ET for aBC and no previous systemic chemotherapy for advanced disease. KISQALI® 600 mg or placebo orally once daily
(3 weeks on/1 week off)+ AI (letrozole 2.5 mg continuous). The primary endpoint was locally assessed PFS, and the key secondary endpoint was OS. Other secondary endpoints included the ORR (complete or partial response), the CBR (overall response plus stable disease lasting 24 weeks or more), safety, and QoL assessments.5,6
MONALEESA-3: N=726, double-blind, placebo-controlled, 2:1 randomised, phase III trial. As 1L and 2L in advanced disease plus those with early relapse in postmenopausal women with HR+/HER2– aBC. KISQALI® 600 mg or placebo orally once daily (3 weeks on/1 week off) + 500 mg intramuscular fulvestrant. The primary endpoint was locally assessed PFS. Secondary endpoints included OS, ORR, CBR, safety and tolerability. 1L defined as: Newly diagnosed (de novo) aBC patients or patients with relapse >12 months from completion of neoadjuvant ET with no treatment for aBC or metastatic disease. 2L was defined as: Relapse on or within 12 months from completion of neoadjuvant ET with no treatment for advanced or metastatic disease (early relapse); relapse >12 months from completion of neoadjuvant therapy with subsequent progression after one line of ET for advanced or metastatic disease, and advanced or metastatic breast cancer at diagnosis that progressed after one line of ET for advanced disease with no prior neoadjuvant treatment for early disease.7,8
MONALEESA-7: N=672, double-blind, placebo-controlled, 1:1 randomised, phase III trial in pre- or perimenopausal women with HR+/HER2− aBC. As 1L in advanced disease and in patients who received 1 or fewer lines of chemotherapy for aBC. KISQALI® 600 mg or placebo orally once daily (3 weeks on/1 week off) + AI (letrozole 2.5 mg oranastrozole 1 mg) or tamoxifen* 20 mg orally once daily continuously + LHRH agonist (goserelin 3.6 mg subcutaneously on day 1 of every cycle). The primary endpoint was investigator-assessed PFS. The key secondary endpoint was OS, defined as the time from randomisation to death from any cause.9
KISQALI® should not be co-administered with tamoxifen.1
1L, first-line; 2L, second-line; aBC, advanced breast cancer; AEs, adverse events; AI, aromatase inhibitor; CBR, clinical benefit rate; ET, endocrine therapy;
HER2-, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive; LHRH, luteinising hormone-releasing hormone;
ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QoL, quality of life; QTcF, QT interval (Fridericia formula).
KISQALI® NSS - UAE
KISQALI® NSS - UAE
References
KISQALI (ribociclib). Prescribing Information.
Borstnar S, et al. Radiol Oncol. 2022;65(2):238–247.
Jackisch C, et al. Poster presentation P4-01-01. Presented at San Antonio Breast Cancer Symposium 2022, 6–10 December, San Antonio, USA.
Burris HA, et al. Br J Cancer. 2021;125:679–686.
Hortobagyi GN, et al. N Engl J Med. 2022;386(10):942–950.
Hortobagyi GN, et al. N Engl J Med. 2016;375(18):1738–1748.
Neven P, et al. Breast Cancer Res. 2023;25:103.
Slamon DJ, et al. J Clin Oncol. 2018;36(24):2465–2472.
Lu Y-S, et al. Clin Cancer Res. 2022;28:851–859.
