The preferred choice
KISQALI® is the preferred CDK4/6i in patients with
HR+/HER2– aBC1-3
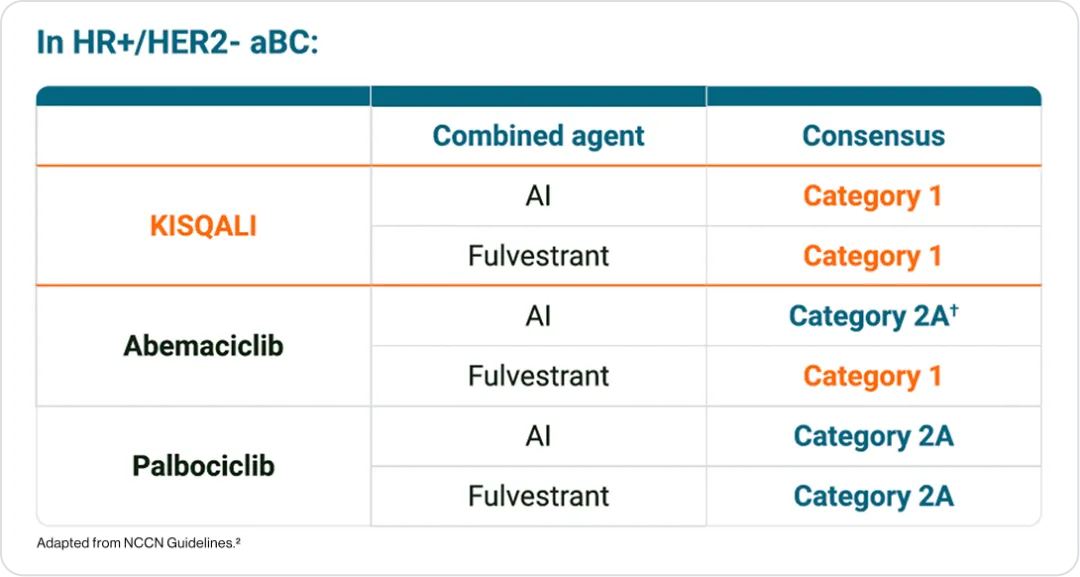
NCCN guidance
KISQALI® is the only category 1** rated CDK4/6i in combination with both AI and fulvestrant2
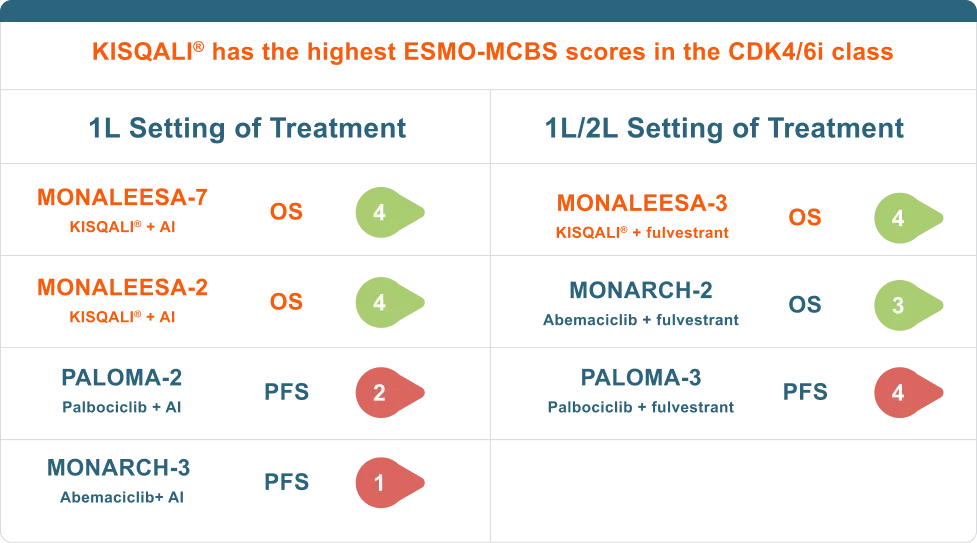
OS achieved
KISQALI® Is the only CDK4/6i to achieve mOS in combination with an AI or fulvestrant in patients with HR+ HER2- mBC3-6
*Other CDK4/6 is remain options based on patient comorbidities, tolerance, and availability.¹
**Based upon high-level evidence (≥1 randomised phase III trials or high-quality, robust meta-analyses), there is uniform NCCN consensus (≥85% support of the panel) that the intervention is appropriate.²
†Based upon lower-level evidence, there is uniform NCCN consensus (≥85% support of the panel) that the intervention is appropriate.²
‡Compared with ET alone.
aBC, advanced breast cancer; ABC6/7, advanced breast cancer guidelines 6 and 7; AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitor; ESMO, European Society for Medical Oncology; HER2-, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive;
MCBS, Magnitude of Clinical Benefit Scale; NCCN, National Comprehensive Cancer Network; OS, overall survival; PFS, progression-free survival; 1L, first-line; 2L, second-line.
KISQALI® NSS - UAE
KISQALI® NSS - UAE
References
Cardoso F, et al. Breast. 2024;76:103756.
NCCN Guidelines® Insights. BreastCancer.
ESMO. ESMO-MCBS scorecards last accessed on July 2025.
Im SA, et al. N Eng J Med. 2019;381:307–316.
Hortobagyi GN, et al. NEJM. 2022;386:942–950.
Neven P, et al. Breast Cancer Research. 2023;25:103.