

Efficacy of KISQALI® (ribociclib) in early breast cancer (eBC)
Indications:1
KISQALI in combination with an aromatase inhibitor (AI), is indicated for the adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer at high risk of recurrence (see section 5.1 of the SmPC for eligibility criteria)
In pre- or perimenopausal women, or in men, the AI should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
KISQALI is not recommended to be used in combination with tamoxifen.1
For more safety information, click here. For the full safety profile, please refer to the KISQALI Summary of Product Characteristics.
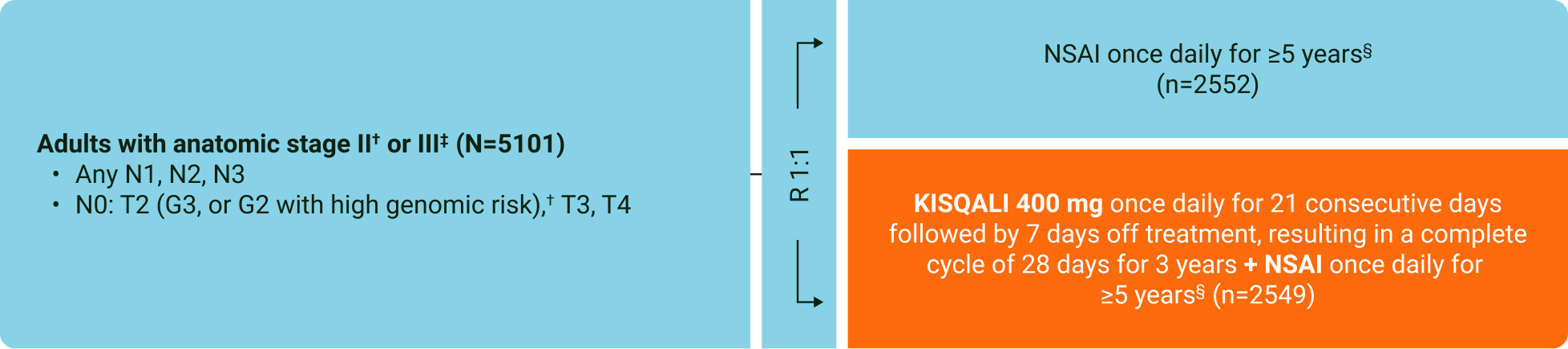
The NATALEE trial included a wide range of patients with HR+/HER2− eBC*2,3
Progress has been made towards reducing recurrence in patients with eBC.4 Until recently, only a minority of HR+/HER2− eBC patients were eligible for targeted treatments.5,6
To help address this unmet need, the multicentre, randomised, open-label NATALEE trial enrolled a range of patients, including those with high-risk N0 disease.3
NATALEE study design:3,7
KISQALI 400 mg dose (vs 600 mg in aBC) selected to help improve tolerability, and potentially, adherence3
In HR+/HER2− eBC, KISQALI + NSAI prevented 1 in 4 invasive disease, recurrence or death events over 3 years vs NSAI7
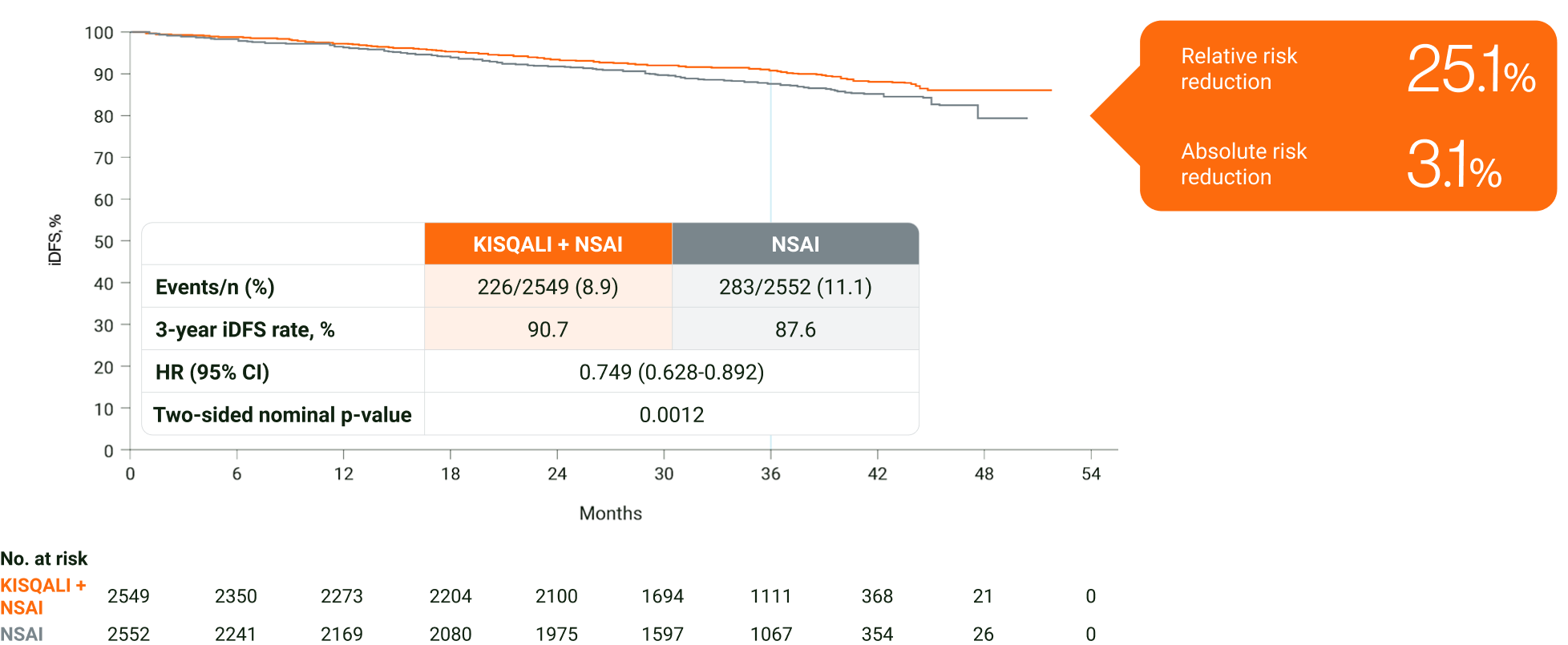
Adapted from Hortobagyi GN, et al. 2024.7
The primary endpoint of the study was met at the primary analysis (cutoff: 11 January 2023). A statistically significant improvement in iDFS (HR=0.748; 95% CI: 0.618–0.906; one-sided stratified log-rank test p=0.0014) was demonstrated in patients receiving KISQALI + AI over AI alone. The results above are those of the pre-specified final analysis (cutoff: 21 July 2023) and should not be regarded as statistically significant. Nominal p values are displayed.1,8
In an exploratory analysis in HR+/HER2− eBC, KISQALI + NSAI was observed to reduce the risk of invasive disease, recurrence or death by 28.5% over 4 years vs NSAI (ARR=4.9%)2
In HR+/HER2− eBC, KISQALI + NSAI prevented 1 in 4 DDFS events at 3 years vs NSAI∥7
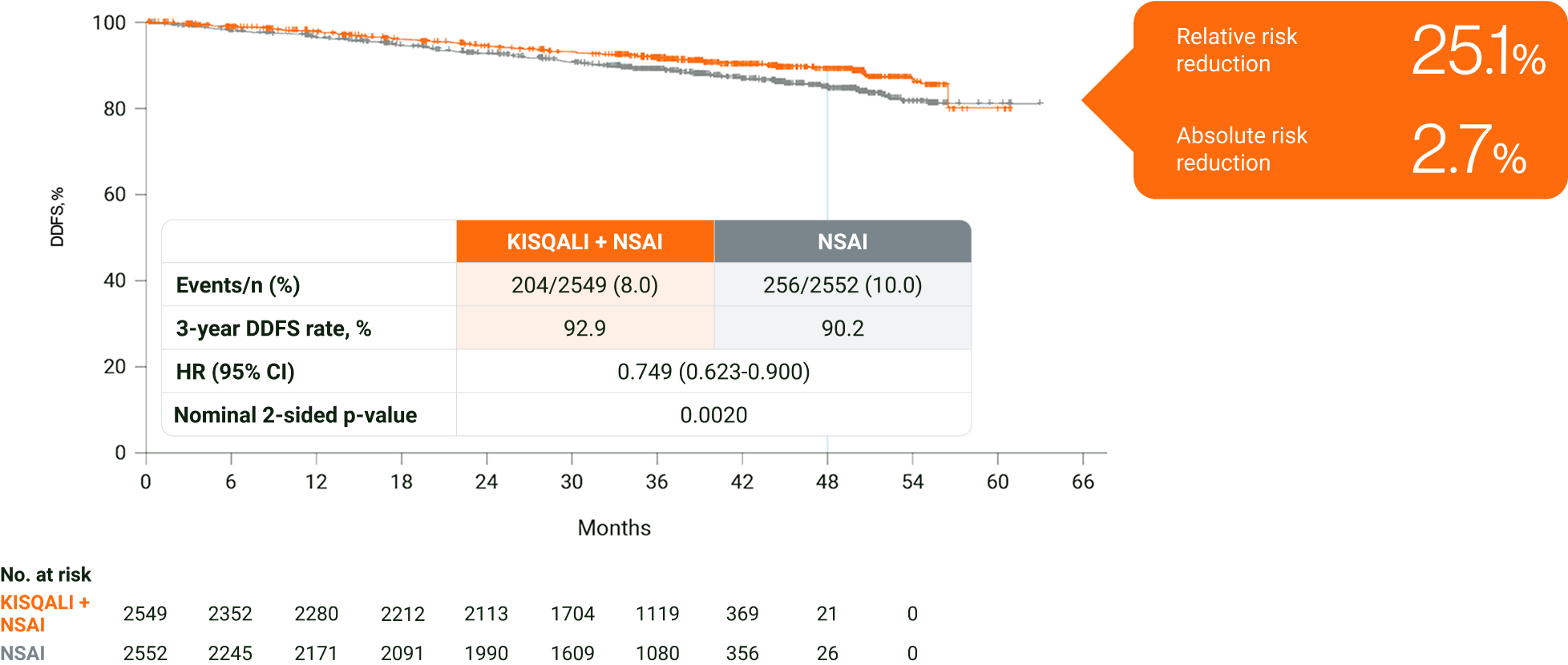
Adapted from Hortobagyi GN, et al. 2024.7
The results above are from the protocol-specified final analysis (cutoff: 21 July 2023) and should not be regarded as statistically significant. Nominal p values have been provided.7
In an exploratory analysis in HR+/HER2− eBC, KISQALI + NSAI was observed to prevent >1 in 4 DDFS events over 4 years vs NSAI (ARR=2.8%)2
Within 5 years, approximately up to 12% of patients with HR+/HER2− eBC may experience a recurrence, despite SOC.9 Across two trials in HR+ eBC, ~75% of BC recurrences were shown to be incurable metastases, despite SoC.¶4,10
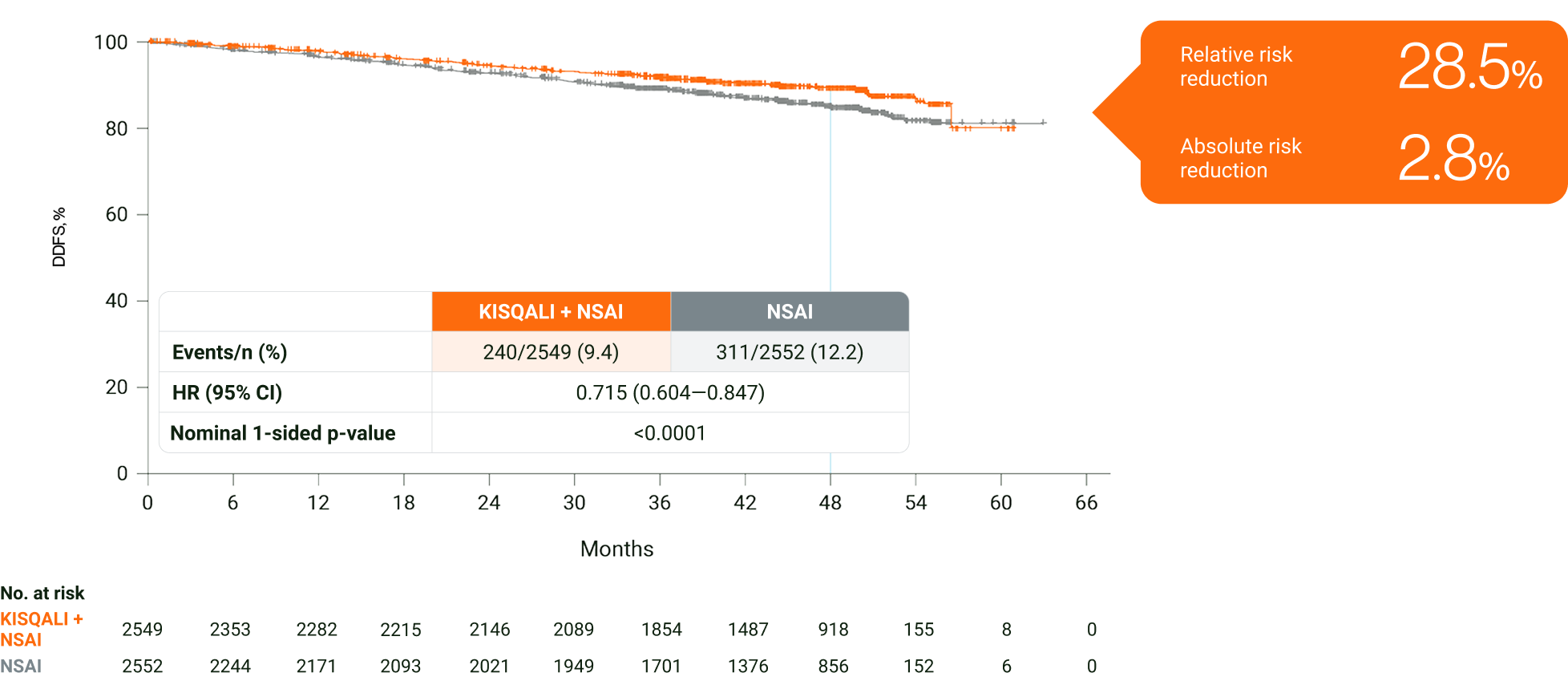
In an exploratory analysis in HR+/HER2− eBC, KISQALI + NSAI was observed to reduce the risk of invasive disease, recurrence or death over 4 years vs NSAI2
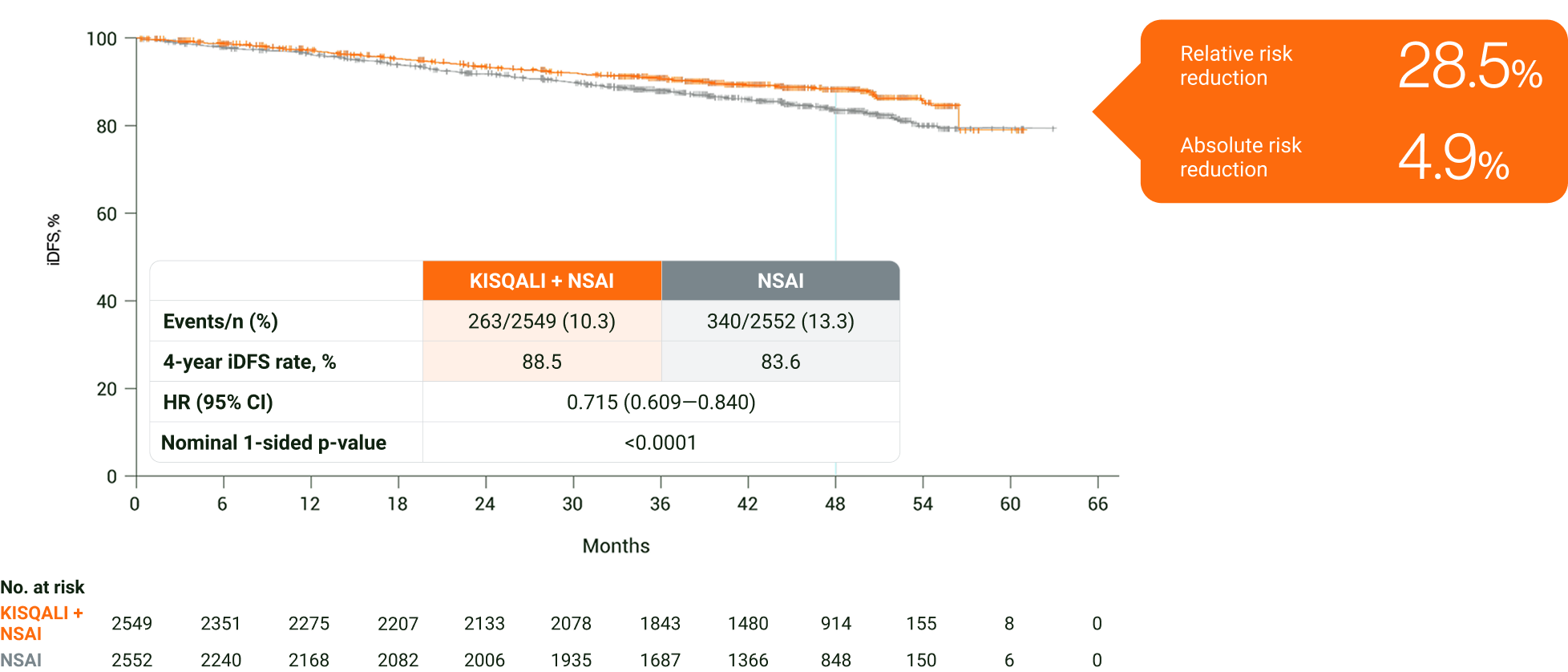
Adapted from Fasching PA, et al. 2024.2
The results above are those of an exploratory analysis (cutoff: 29 April 2024) and should not be regarded as statistically significant. Nominal p values have been provided.2
In an exploratory analysis in HR+/HER2− eBC, KISQALI + NSAI was observed to prevent >1 in 4 DDFS events over 4 years vs NSAI2
In NATALEE, DDFS was a secondary endpoint.2
Adapted from Fasching PA, et al. 2024.2
The results above are from an exploratory analysis (cutoff: 29 April 2024) and should not be regarded as statistically significant. Nominal p values have been provided.
Summary of the safety profile
The most common ADRs (reported at a frequency ≥20%) in the dataset for which the frequency for KISQALI + AI exceeds the frequency for AI alone were neutropenia, infections, nausea, headache, fatigue, leukopenia and abnormal liver function tests. The most common grade 3/4 ADRs (reported at a frequency of ≥2%) in the dataset for which the frequency for KISQALI + AI exceeds the frequency for AI alone were neutropenia, abnormal liver function tests and leukopenia.1
NATALEE: The most common AEs of any grade were neutropenia (62.5% with KISQALI + NSAI vs 4.6% with NSAI alone), arthralgia (37.3% vs 43.3%, respectively), and nausea (23.3% vs 7.8%, respectively). The most common grade ≥3 event was neutropenia (44.3% with KISQALI + NSAI vs 0.9% with NSAI alone). Grade ≥3 liver-related AEs occurred in 8.6% of patients in the KISQALI + NSAI arm and 1.7% in the NSAI-alone arm.7
Learn about the safety profile of KISQALI in eBC
Learn about the efficacy of KISQALI in aBC
*Key inclusion criteria: Patients in NATALEE were diagnosed up to 18 months prior to randomisation with HR+/HER2− eBC. Randomisation was stratified by menopausal status, anatomic stage II or III, prior (neo)adjuvant CT and geographic region. Permitted to have already received any standard (neo)adjuvant ET, but must be randomised within 12 months of the initial start date of ET. Key exclusion criteria: distant metastases of BC beyond the regional lymph nodes and/or evidence of disease after curative surgery; prior treatment with CDK4/6i; prior treatment with anthracyclines at cumulative doses of ≥450 mg/m2 for doxorubicin or ≥900 mg/m2 for epirubicin; major surgery, CT or radiotherapy within 14 days prior to randomisation; prior treatment with tamoxifen, raloxifene or AIs for reduction in risk of BC and/or prior treatment for osteoporosis in the preceding 2 years.3
†Anatomic stage IIA inclusive of N0 (with grade 2 and evidence of high risk: Ki-67 ≥20%, Oncotype DX Breast Recurrence Score ≥26, or high risk via genomic risk profiling) or grade 3 and N1, and anatomic stage IIB inclusive of N0 or N1. Enrollment of patients with stage II capped at 40%.3
‡Anatomic stage III inclusive of N0, N1, N2, or N3.3
§Men and premenopausal women also received goserelin.3
∥Distant disease-free survival is defined as the time from date of randomisation to date of first event of distant recurrence, death (any cause), or second primary non-breast invasive cancer.3
¶Based on results from 2 trials: Meta-analysis by the EBCTCG of AIs vs tamoxifen in 7030 premenopausal women with ER+ eBC. In both treatment arms at 0–4 years, 74% of recurrences were metastatic (423/569 recurrence events). Data from the Phase III randomised BIG 1-98 trial of letrozole and tamoxifen in post-menopausal women with HR+ eBC, N=8010. In both treatment arms at 5 years, 76% of recurrences were metastatic (409/535 recurrences).4,10
aBC, advanced breast cancer; ADR, adverse drug reaction; AI, aromatase inhibitor; ARR, absolute risk reduction; BC, breast cancer; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; CT, chemotherapy; CI, confidence interval; DDFS, distant disease-free survival; eBC, early breast cancer; EBCTCG, Early Breast Cancer Trialists’ Collaborative Group; ER, oestrogen receptor; ET, endocrine therapy; G, grade; HER2−, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HR+, hormone receptor-positive; HRQoL, health-related quality of life; iDFS, invasive disease-free survival; LHRH, luteinising hormone-releasing hormone; N0, no nodal involvement; N1, 1–3 axillary lymph nodes; N2, 4–9 axillary lymph nodes; N3, ≥10 axillary lymph nodes or collarbone lymph nodes; NSAI, non-steroidal aromatase inhibitor; OS, overall survival; R, randomisation; SoC, standard of care; STEEP, standardised definitions for efficacy endpoints; T, tumour; T2, tumour is more than 2 cm but less than 5 cm; T3, tumour is more than 5 cm; T4, tumour of any size growing into the chest wall or skin, includes inflammatory breast cancer.
References
KISQALI (ribociclib) Summary of Product Characteristics.
Fasching PA, et al. Oral LBA13. European Society for Medical Oncology Congress 2024, 13–17 September 2024, Barcelona, Spain.
Slamon DJ, et al. Ther Adv Med Oncol 2023;15:1–16.
The Breast International Group 1-98 Collaborative Group. N Engl J Med 2005;353:2747–2757.
Verzenios (abemaciclib) Summary of Product Characteristics.
Lynparza (olaparib) Summary of Product Characteristics.
Hortobagyi GN, et al. Ann Oncol 2024;36(2):149-157.
Slamon DJ, et al. N Engl J Med 2024;390:1080–1091.
Curigliano G, et al. J Clin Oncol 2024;42(Suppl 16): abstract 541.
The Early Breast Cancer Trailists’ Collaborative Group. Lancet Oncology 2022;23:382–392.
UK | July 2025 | FA-11344815
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.