

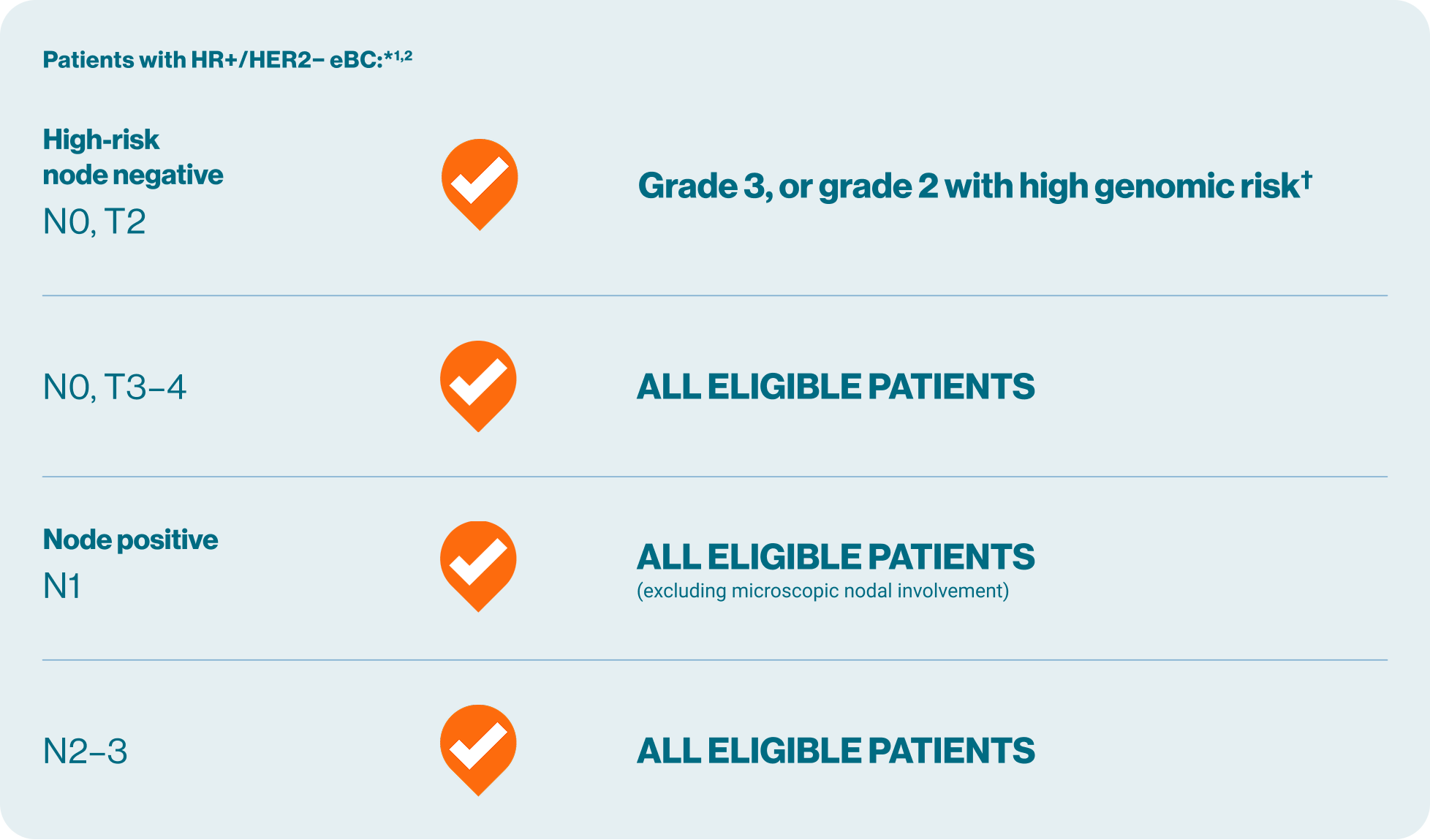
Patient eligibility for KISQALI® (ribociclib) in early breast cancer (eBC)
Indications:1
KISQALI in combination with an aromatase inhibitor (AI), is indicated for the adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer at high risk of recurrence (see section 5.1 of the SmPC for selection criteria)
In pre- or perimenopausal women, or in men, the AI should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
KISQALI is not recommended to be used in combination with tamoxifen.
Please refer to the Summary of Product Characteristics (SmPC) for the full safety profile.
Now you can offer KISQALI + AI to a broad range of your eligible HR+/HER2− eBC patients*1,2
Explore the efficacy outcomes of KISQALI in eBC
Learn about the safety profile of KISQALI in eBC
*KISQALI in combination with an AI is indicated for the adjuvant treatment of patients with hormone receptor HR+/HER2– eBC at high risk of recurrence. This includes patients with lymph node-positive cancer (excluding microscopic nodal involvement), or if no nodal involvement either tumour size >5 cm, or tumour size 2–5 cm with either grade 2 and high genomic risk or Ki67 ≥20%, or grade 3. AI should be combined with an LHRH agonist.1
†High genomic risk includes Ki67 ≥20% or high risk by gene signature testing.1,2
AI, aromatase inhibitor; eBC, early breast cancer; HER2−, human epidermal growth factor receptor 2-negative; HR+, hormone receptor-positive; LHRH, luteinising hormone-releasing hormone; N0, no nodal involvement; N1, 1–3 axillary lymph nodes; N2, 4–9 axillary lymph nodes; N3, ≥10 axillary lymph nodes or collarbone lymph nodes; NSAI, non-steroidal aromatase inhibitor; T, tumour; T2, tumour is more than 2 cm but less than 5 cm; T3, tumour is more than 5 cm; T4, tumour of any size growing into the chest wall or skin, includes inflammatory breast cancer.
References
KISQALI (ribociclib) Summary of Product Characteristics.
Slamon DJ, et al. Ther Adv Med Oncol 2023;15:1–16.
UK | July 2025 | FA-11344842
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.