ILARIS® (canakinumab) efficacy data
Indications1
Periodic fever syndromes
ILARIS is indicated for the treatment of the following autoinflammatory periodic fever syndromes in adults, adolescents and children aged 2 years and older:
Cryopyrin-associated periodic syndromes (CAPS), including:
Muckle-Wells syndrome (MWS)
Neonatal-onset multisystem inflammatory disease (NOMID)/chronic infantile neurological, cutaneous, articular syndrome (CINCA)
Severe forms of familial cold autoinflammatory syndrome (FCAS)/familial cold urticaria (FCU) presenting with signs and symptoms beyond cold-induced urticarial skin rash
Tumour necrosis factor receptor associated periodic syndrome (TRAPS)
Hyperimmunoglobulin D syndrome (HIDS)/mevalonate kinase deficiency (MKD)
Familial Mediterranean Fever (FMF)
ILARIS should be given in combination with colchicine, if appropriate
Still’s disease
ILARIS is indicated in patients who have responded inadequately to previous therapy with non-steroidal anti-inflammatory drugs (NSAIDs) and systemic corticosteroids, for the treatment of active Still’s disease, including:
Adult-onset Still’s disease (AOSD)
Systemic juvenile idiopathic arthritis (SJIA) in patients aged 2 years and older
ILARIS can be given as monotherapy or in combination with methotrexate
Gouty arthritis
ILARIS is indicated for the symptomatic treatment of adult patients with frequent gouty arthritis attacks (at least 3 attacks in the previous 12 months) in whom non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine are contraindicated, are not tolerated, or do not provide an adequate response, and in whom repeated courses of corticosteroids are not appropriate.
Summary of the safety profile1
The most frequent adverse reactions were infections predominantly of the upper respiratory tract. No impact on the type or frequency of adverse reactions was seen with longer-term treatment.
Very common adverse reactions (reported ≥1/10 patients) were respiratory tract infections (including pneumonia, bronchitis, influenza, viral infection, sinusitis, rhinitis, pharyngitis, tonsillitis, nasopharyngitis, upper respiratory tract infection), ear infection, cellulitis, gastroenteritis, urinary tract infection, upper abdominal pain, injection site reaction, arthralgia, creatinine renal clearance decreased, proteinuria, leukopenia.
For full safety information, please refer to the ILARIS Summary of Product Characteristics (SmPC).
For more safety information in periodic fever syndromes (PFS), click here.
For more safety information in Still’s disease, click here.
Posology1
The recommended starting dose of ILARIS in TRAPS, HIDS/MKD and FMF patients is 150 mg for patients with body weight >40 kg or 2 mg/kg for patients with body weight ≥7.5 kg and ≤40 kg, every four weeks as a single dose via subcutaneous injection.
If a satisfactory clinical response has not been achieved 7 days after treatment start, a second dose of ILARIS at 150 mg or 2 mg/kg can be considered. If a full treatment response is subsequently achieved, the intensified dosing regimen of 300 mg (or 4 mg/kg for patients weighing ≤40 kg) every 4 weeks needs to be maintained.
In patients without clinical improvement, it is recommended that the treating physician reconsiders continued treatment with ILARIS.
For more information, please refer to the SmPC.
Epoch 1
Screening: 12-week screening period to confirm study eligibility; patients entered Epoch 2 when they experienced a flare (PGA score ≥2 and CRP >10 mg/L)Epoch 2
Randomised treatment or placebo for 16 weeks. Patients received SC ILARIS 150 mg (or 2 mg/kg)* or placebo every 4 weeks. All patients were eligible for a blinded dose increase (to ILARIS 300 mg SC) if they had a persistent baseline flare between Days 8 and 14 (PGA score ≥2, or CRP >10 mg/L with <40% reduction from baseline) or a lack of resolution at Day 15 (resolution was defined as a PGA score <2 plus a CRP ≤10 mg/L or a reduction by ≥70% from baseline). After Day 29, patients were eligible for an open-label increase in the dose if they had a flare (PGA score ≥2 and CRP ≥30 mg/L)Epoch 3
Randomised withdrawal and open-label period of 24 weeks†Epoch 4
72-week open-label treatment extension period for patients treated with ILARISAIDAI
AIDAI is a novel and unique, validated patient-reported assessment tool to evaluate disease activity in FMF, HIDS/MKD and TRAPS. This study performed an external validation of AIDAI by calculating scores over 40 weeks of ILARIS treatment in patients enrolled on the CLUSTER trial. Overall, 167 patients (crFMF: n=59; HIDS/MKD: n=66; TRAPS: n=42) were randomised to ILARIS 150 mg or placebo every 4 weeks3Part 1
An open-label treatment period in which a single dose of ILARIS was administered. Response was assessed during the following 8 weeksPart 2
A double-blind withdrawal period in which patients who had a sustained complete response in part 1 were randomly assigned to receive either ILARIS or placebo every 8 weeks for up to 24 weeksPart 3
At the end of part 2 or at the time of relapse, whichever occurred first, patients immediately entered the open-label part 3 of the study, in which they received ILARIS every 8 weeks for a minimum of 16 weeks, for a total study duration of 48 weeks
Key efficacy data for ILARIS in the treatment of PFS (FMF, HIDS/MKD and TRAPS)
ILARIS may help FMF, HIDS/MKD and TRAPS patients to achieve a rapid and complete response2
At Week 16, significantly more patients receiving ILARIS had achieved the primary endpoint of a complete response vs placebo.2
Complete response was defined as resolution of the baseline flare at Day 15 (PGA score <2 plus CRP ≤10 mg/L or a reduction by ≥70% from baseline) and no new flare (PGA score ≥2 and CRP ≥30 mg/L) up to Week 16.2
Proportion of patients who had a complete response at Week 162
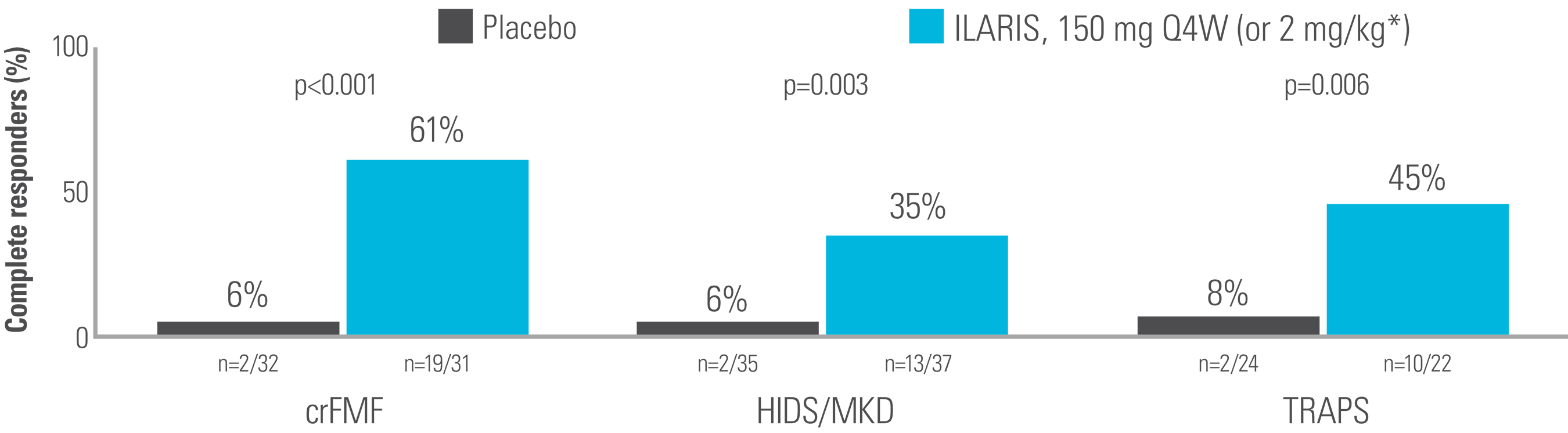
Adapted from De Benedetti et al. 2018.2
In an exploratory analysis including those who received a blinded dose increase of ILARIS to 300 mg every 4 weeks before Day 29, significantly more patients achieved a complete response with ILARIS vs placebo at Week 16.2
Proportion of patients (including 150 mg Q4W blindly up-titrated to 300 mg Q4W) who had a complete response at Week 162
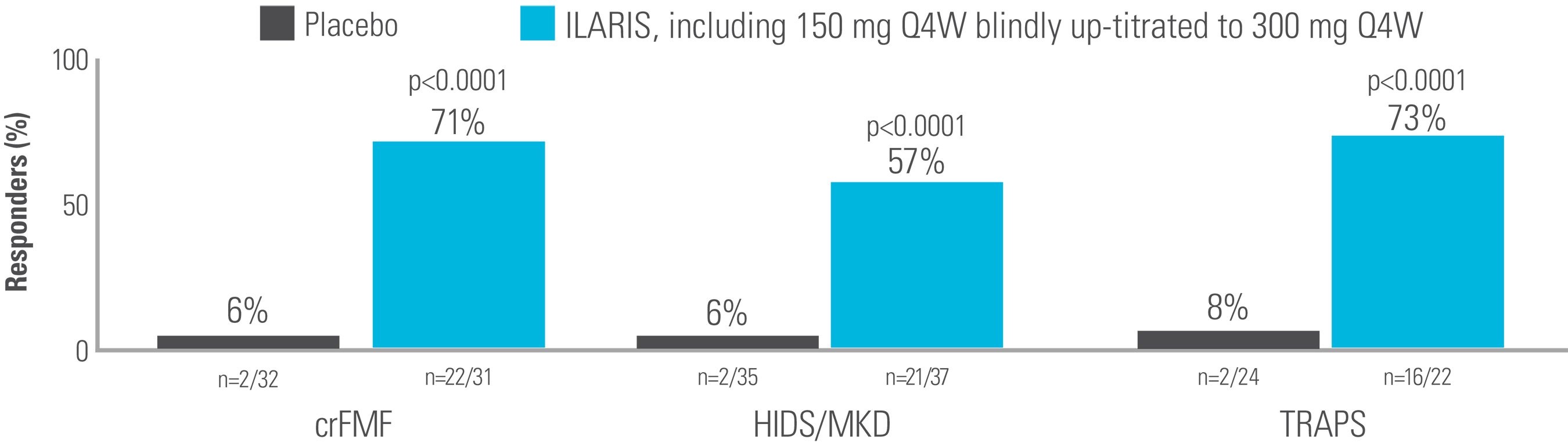
Adapted from supplement to De Benedetti et al. 2018.2
In an exploratory analysis, AIDAI scores decreased over 40 weeks of treatment with ILARIS in patients with FMF, HIDS/MKD and TRAPS3
Median AIDAI scores over 40 weeks3
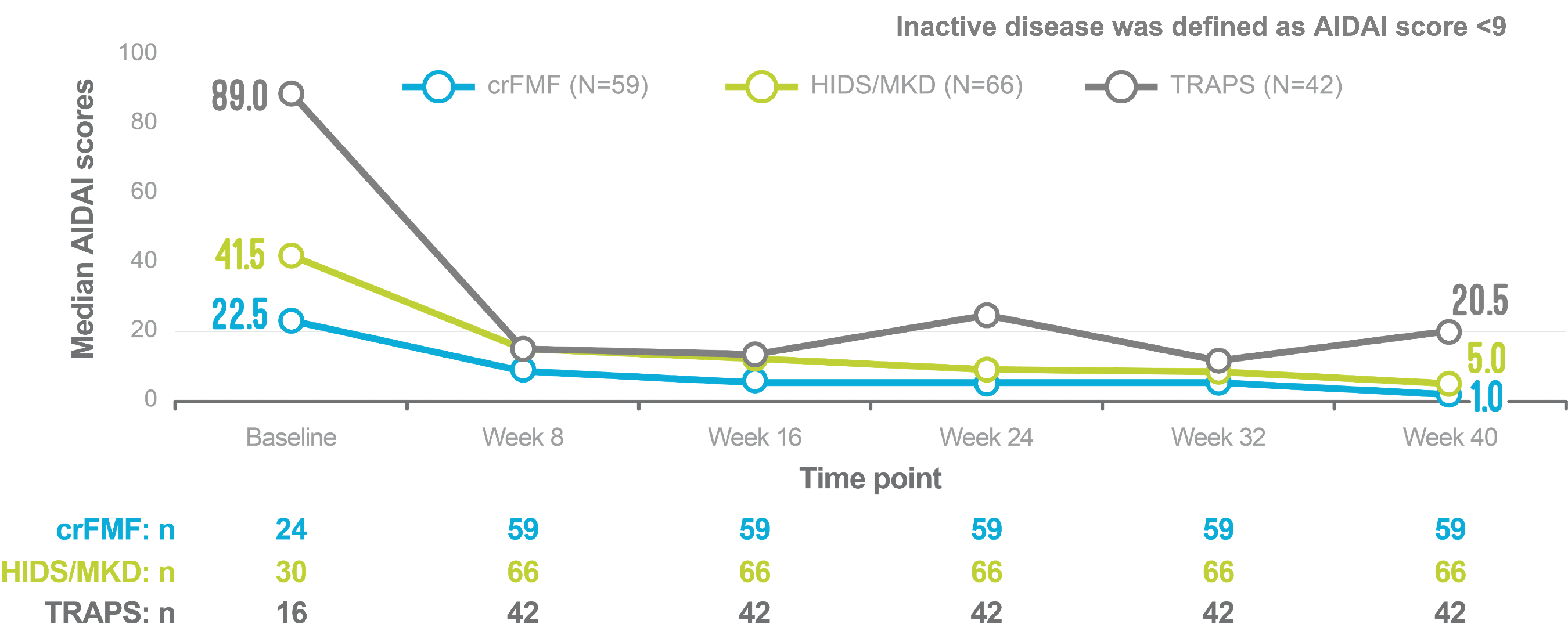
Adapted from Koné-Paut I, et al. 2018.3
Study design
CLUSTER study2
Randomised, double-blind, placebo-controlled study (N=181) to evaluate the efficacy and safety profile of ILARIS in patients with crFMF (n=63), HIDS/MKD (n=72) and TRAPS (n=46). The trial consisted of one cohort per disease. Each cohort followed the same study design:
Primary outcome was the proportion of patients who had a complete response. The secondary outcomes were the proportion of patients who had a PGA score of <2, a CRP level ≤10 mg/L, or a serum amyloid A (SAA) level≤10 mg/L at Week 16 and, in epoch 3, the proportion of patients receiving ILARIS or placebo every 8 weeks who had no flare.2
The safety profile and efficacy of ILARIS in CAPS, TRAPS, HIDS/MKD and FMF patients under 2 years of age have not been established. Currently available data are described in the SmPC but no recommendation on a posology can be made.1
*2 mg/kg for patients weighing ≤40 kg.2
†For some patients in the CLUSTER study, the timing of dose up-titration or the frequency of ILARIS administration differed from the recommendations of the SmPC. Please refer to the Prescribing Information or the ILARIS SmPC for information on posology.1
Key efficacy data for ILARIS in the treatment of PFS (CAPS)
Note: The population of patients aged 28 days to less than 2 years enrolled in the study by Brogan et al 2019 is off-label. The safety profile and efficacy of canakinumab in CAPS, TRAPS, HIDS/MKD and FMF patients under 2 years of age have not been established. Currently available data are described in sections 4.8, 5.1 and 5.2 of the SmPC but no recommendation on a posology can be made.
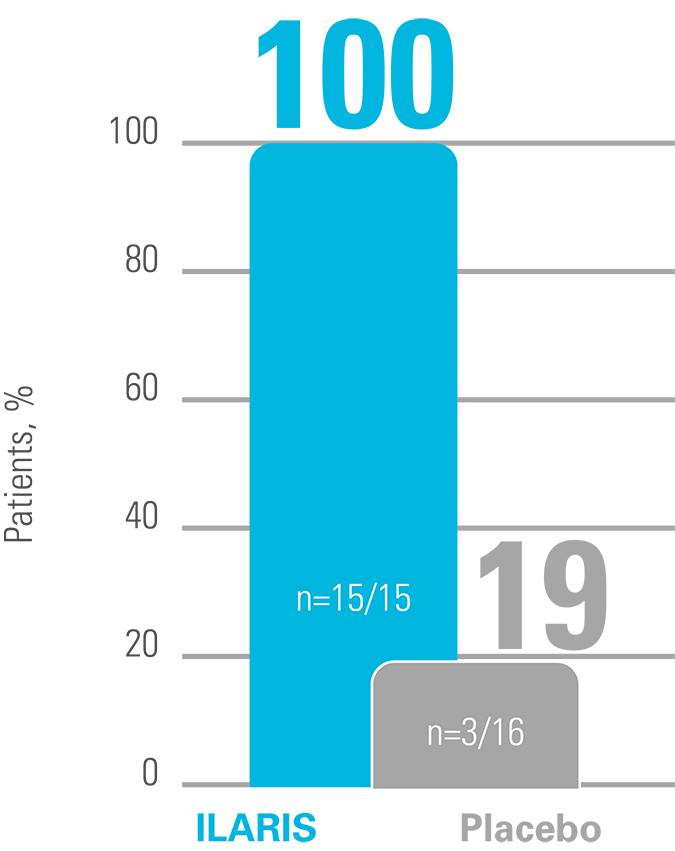
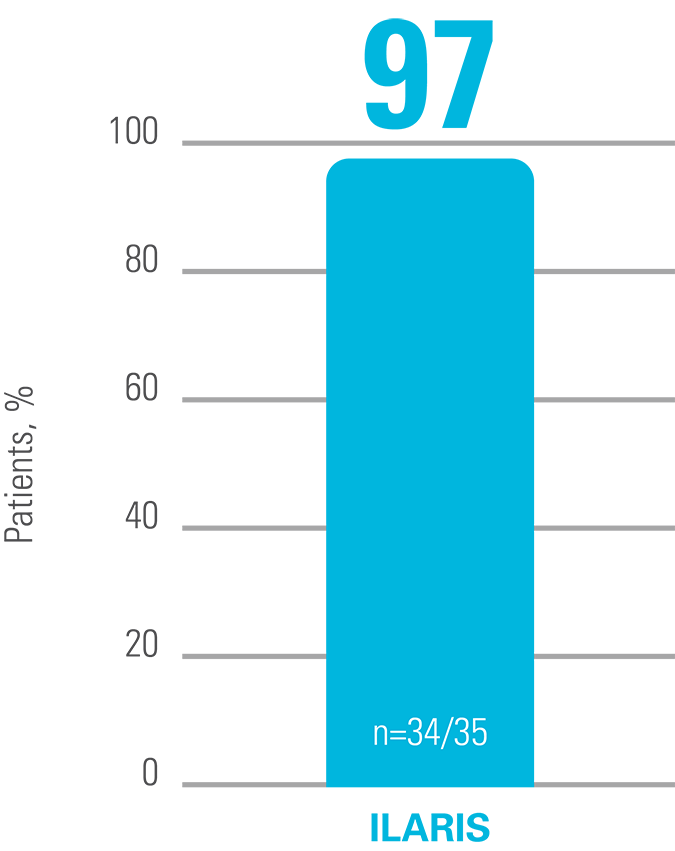
ILARIS may help CAPS patients to achieve a rapid and complete response4
A complete response was defined as a PGA of no or minimal disease activity, an assessment of no or minimal rash, and a value for both serum CRP and SAA that was within the normal range (<10 mg/L for both measures). PGA of disease activity was based on a composite of the following symptoms: urticarial skin rash, arthralgia, myalgia, headache/migraine, conjunctivitis, fatigue and malaise.4
Proportion of patients who did not have a flare in the 24-week ILARIS withdrawal phase4
Adapted from Lachmann HJ, et al. 2009.4
Proportion of patients who achieved a complete response in the initial open-label phase (observed data)4
Adapted from Lachmann HJ, et al. 2009.4
Relapse data in CAPS patients treated with ILARIS (observed data)5
In a 2-year, open-label study of 166 CAPS patients treated with ILARIS (observed data):5
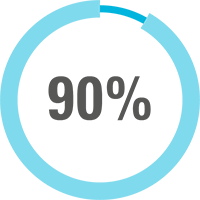
of patients (n=141) maintained on ILARIS* were relapse free.†
In a 152-week, open-label extension study of 17 CAPS patients treated with ILARIS (observed data):6

of patients (n=17) achieved a complete response within 72 weeks, and no new relapses‡ occurred after Week 96.
Study designs
REMITTER4
Multicentre, randomised, double-blind, placebo-controlled study of ILARIS for the treatment of CAPS (N=35). Eligible patients (aged 4–75 years, weighing ≥15–<100 kg) had CAPS associated with an NLRP3 mutation and required treatment (n=35). Previous treatment with anakinra, rilonacept or ILARIS was permitted if treatment had been discontinued and the patient’s disease had relapsed. The study consisted of three parts:
The primary outcome measure was the proportion of patients with a relapse of CAPS during treatment with ILARIS, as compared with placebo, in the withdrawal phase (part 2). Secondary outcome measures included the proportion of patients with a complete response in part 1, values of inflammatory markers, global assessments by physicians and patients, and safety and tolerability profiles.
Kuemmerle-Deschner et al. 20115
Open-label, Phase III study of ILARIS for the treatment of CAPS (N=166). Eligible CAPS patients (aged 3 years or older) were either ILARIS-naïve (n=109) or had been pre-treated with ILARIS in a previous study (n=57). Patients received SC ILARIS 150 mg or weight-based 2 mg/kg (body weight ≤40 kg) every 8 weeks for up to 2 years. In the case of residual symptoms, patients were maintained on a more intense dosing regimen (increased dose of SC ILARIS up to 600 mg or 8 mg/kg [body weight ≤40 kg]) and/or increased dosing frequency. Assessment of complete response and relapse included ratings for disease activity, skin rash and CRP and/or SAA.
Brogan et al. 20196
Multicentre, open-label, Phase III, 56-week core study of ILARIS for the treatment of CAPS (N=17) to assess the efficacy of ILARIS with respect to treatment response. Patients who completed the core study entered the long-term extension study, with a minimum treatment duration of 6 months and a maximum treatment duration of 24 months, until 152 weeks. Eligible CAPS patients (aged 28 days to 60 months) had confirmed NLRP3 mutations and weighed ≥2.5 kg. Previous treatment with the anti-IL-1 agents anakinra or rilonacept was permitted provided these were discontinued prior to the baseline visit and the patient demonstrated active disease prior to enrolment. Patients received SC ILARIS 2 mg/kg every 8 weeks for the entire study period (including the core and extension studies). Patients in whom a complete response was not achieved after the first dose of ILARIS, and those who experienced a relapse before the next scheduled dose, were eligible for a stepwise dose up-titration (4 to 6 to 8 mg/kg). The extension study assessed the long-term efficacy and safety profile of ILARIS with respect to relapse in patients who completed the core study.
*Patients received ILARIS 150 mg every 8 weeks; median treatment duration was 414 days.5
†Relapse was defined as a value for either CRP or SAA of more than 30 mg/L, accompanied by a PGA greater than minimal or was minimal and accompanied by a rash that was assessed as more than minimal.4
‡Relapse was defined for complete responders based on clinical features (PGA greater than ‘minimal’ or PGA greater than or equal to ‘minimal’ and assessment of skin disease greater than ‘minimal’) and serologic features (serum CRP >30 mg/L or SAA >30 mg/L).6
Key efficacy data for ILARIS in the treatment of Still’s disease (AOSD)
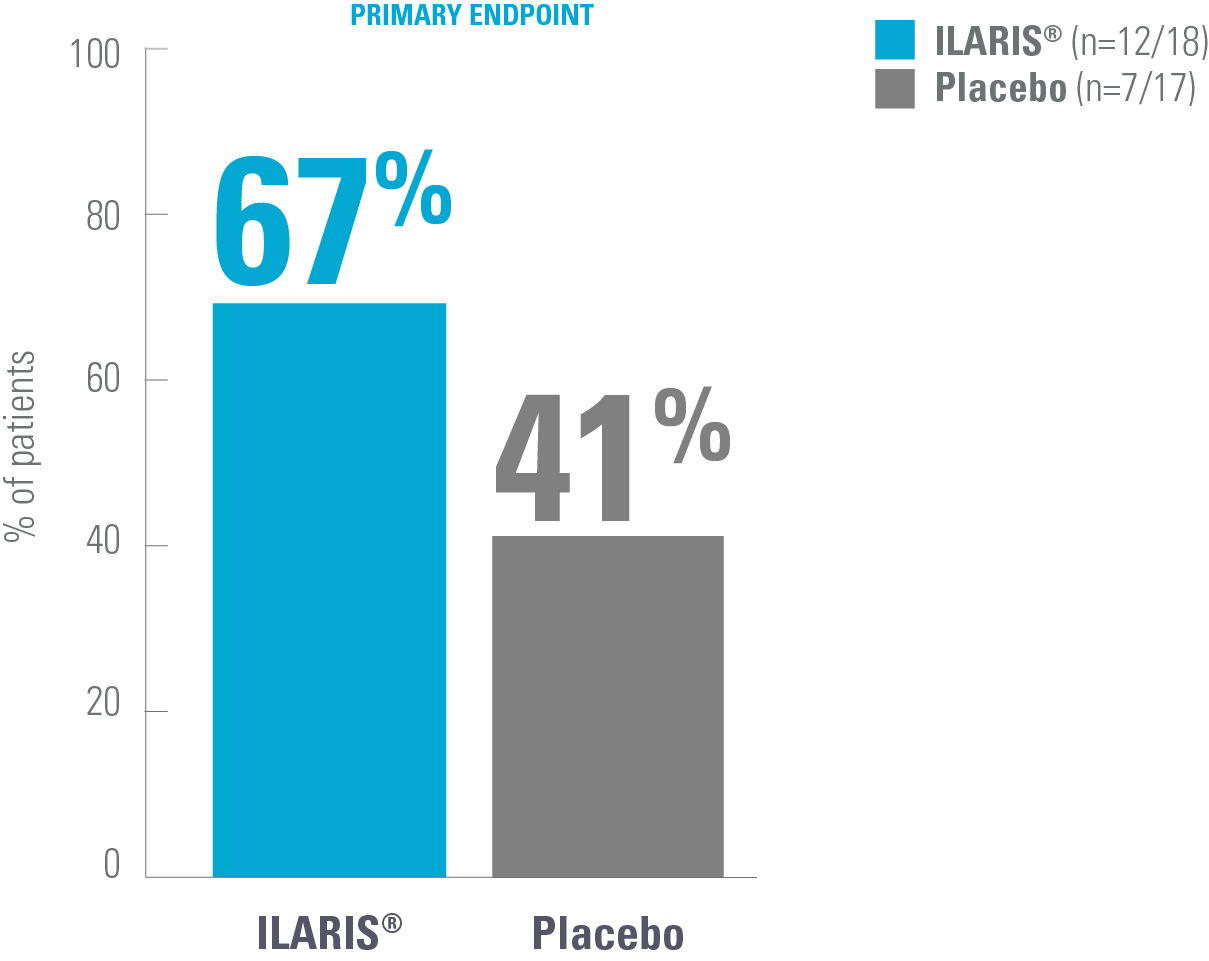
Changes in outcome measures in AOSD with ILARIS vs placebo7
Proportion of patients showing a change from baseline in disease activity at Week 12*7
Adapted from Kedor C, et al. 2020.7
Study design
CONSIDER7
Multicentre, double-blind, randomised, placebo-controlled Phase II trial in patients with AOSD and active joint involvement (tender and swollen joint counts of ≥4 each) (N=36). Patients were treated with SC ILARIS (4 mg/kg, maximum 300 mg every 4 weeks, n=18) or placebo (n=17) (1:1 ratio). The primary endpoint was the proportion of patients with a clinically relevant reduction of the articular manifestation measured by the change in disease activity score (ΔDAS28 >1.2) at Week 12.
*Patients with AOSD and active joint involvement (tender and swollen joint counts of ≥4 each) were treated with SC ILARIS (4 mg/kg, maximum 300 mg every 4 weeks) or placebo.7
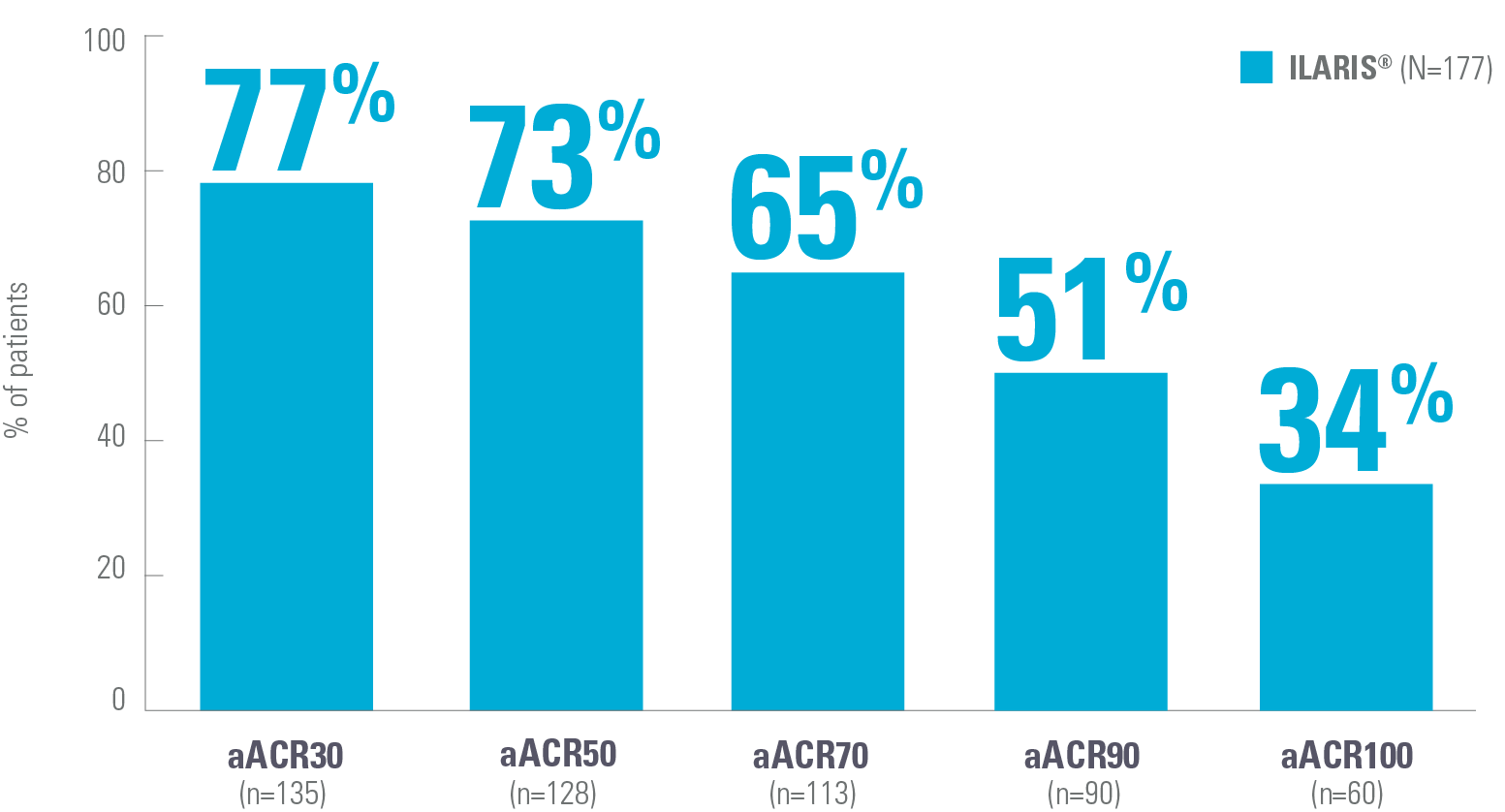
Key efficacy data for ILARIS in the treatment of Still’s disease (SJIA)
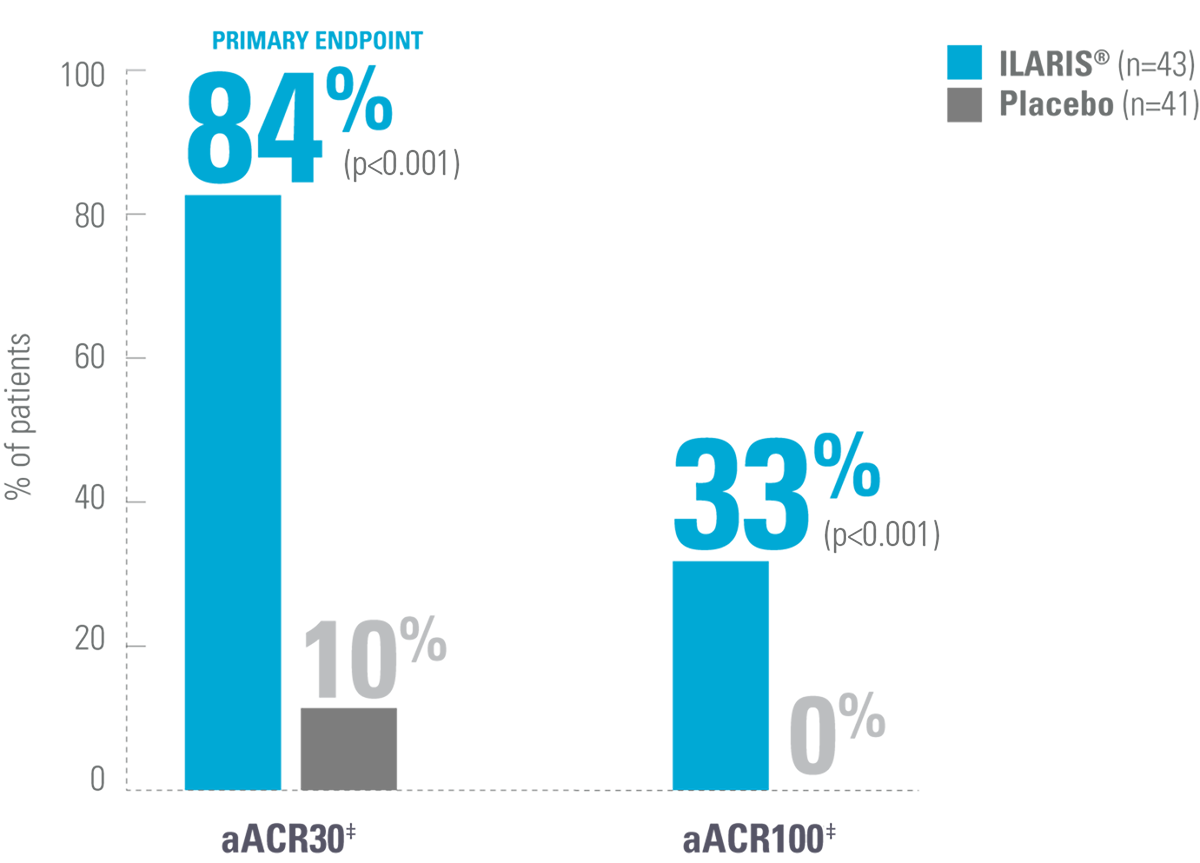
ILARIS may help patients with SJIA achieve fast and lasting disease control8,9
Proportion of patients achieving an aACR30 response by Day 15*9
Adapted from Ruperto N, et al. 2012 (Trial 1).9
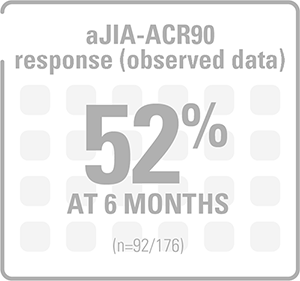
ACR response was maintained after 32 weeks of open-label treatment (observed data)§9
Proportion of patients achieving aACR responses after 32 weeks of open-label treatment (observed data)§9
Adapted from Ruperto N, et al. 2012 (Trial 2).9
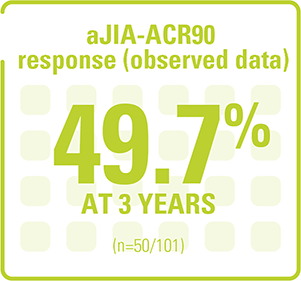
Relief was maintained for up to 3 years in the long-term extension study (observed data, pooled population)¶8
Adapted from Koné-Paut I, et al. 2018.3
Study design
β-SPECIFIC 1 and β-SPECIFIC 28,9
Trial 1 was a randomised, double-blind, placebo-controlled, 4-week Phase III study assessing the short-term efficacy of ILARIS for SJIA (N=84). Patients were randomised to receive a single dose of 4 mg/kg (up to 300 mg) ILARIS (n=43) or placebo (n=41). The primary objective was the proportion of patients who achieved a minimum 30% improvement in the paediatric ACR response criterion adapted to include absence of fever.
Patients from trial 1 could enter the two-part Phase III trial 2 where ILARIS-naïve patients and patients from a Phase II trial were additionally enrolled. Trial 2 of this study enrolled SJIA patients aged 2–19 to receive open-label ILARIS (4 mg/kg up to 300 mg) every 4 weeks for 12–32 weeks. In the open-label phase of trial 2, the primary objective was to determine whether at least 25% of the patients treated with glucocorticoids could have their dose tapered.
After 32 weeks of open-label treatment with ILARIS, patients who had a response and underwent glucocorticoid tapering were randomly assigned to continued treatment with ILARIS or to placebo (withdrawal phase). The primary outcome was time to flare of SJIA.
Long-term extension study of β-SPECIFIC 1 and β-SPECIFIC 28
This study reported the long-term efficacy and safety profile of ILARIS in patients with SJIA with active systemic features and arthritis at baseline, who entered the previously reported pivotal Phase III studies monitored for up to 5 years.
*SJIA patients aged 2–19 were randomised to receive a single dose of either ILARIS (4 mg/kg up to 300 mg) or placebo and followed for 29 days before enrolment to a subsequent trial.9
‡aACR response: Percentage improvement (at least 30%, 50%, 70%, 90%, 100%) in at least 3 of the 6 response variables and worsening of >30% in no more than 1 of the 6 variables, including absence of fever. The disease activity components include CRP level, number of joints with active arthritis, number of joints with limited range of motion, physician’s global assessment of disease activity, parent’s global assessment of patient’s overall well-being and functional ability (CHAQ-DI).9
§In trial 2, the graph shows the rates of aJIA-ACR responses at the end of the 32-week, open-label phase which included data from the last observation of patients who had a response.9
¶Primary analysis included intent-to-treat population from trial 2 based on observed data with all discontinuations at different timepoints counted as missing and with missing data imputed using last observation carried forward. 5-year long-term extension study of the 2 pivotal Phase III trials to evaluate the long-term efficacy and safety profile of ILARIS in SJIA patients. 144 of the 177 patients (81%) enrolled in the core studies entered the long-term extension. 58% (n=102/177) discontinued, with higher discontinuation rates in late responders (81%; n=25/31) vs early responders (29%; n=11/38). No new safety findings were observed.8
aACR, adapted American College of Rheumatology; ACR, American College of Rheumatology; AIDAI, auto-inflammatory diseases activity index; aJIA-ACR, adapted juvenile idiopathic arthritis American College of Rheumatology; AOSD, adult-onset Still’s disease; CAPS, cryopyrin-associated periodic syndromes; CHAQ-DI, childhood health assessment questionnaire–disability index; CINCA, chronic infantile neurological, cutaneous, articular syndrome; crFMF, colchicine-resistant familial Mediterranean fever; CRP, c-reactive protein; DAS28-ESR, disease activity score 28-joint count with erythrocyte sedimentation rate; FCAS, familial cold autoinflammatory syndrome; FCU, familial cold urticaria; FMF, familial Mediterranean fever; HIDS, hyperimmunoglobulin D syndrome; MKD, mevalonate kinase deficiency; MWS, Muckle-Wells syndrome; NLRP3, NLR family pyrin domain containing 3 gene; NOMID, neonatal-onset multisystem inflammatory disease; NSAID, non-steroidal anti-inflammatory drug; PFS, periodic fever syndrome; PGA, physician’s global assessment; Q4W, every 4 weeks; SAA, serum amyloid; SC, subcutaneous; SJIA, systemic juvenile idiopathic arthritis; SmPC, summary of product characteristics; TRAPS, tumour necrosis factor receptor-associated periodic syndrome.
Reference
ILARIS® (canakinumab) Summary of Product Characteristics.
De Benedetti F, et al. N Engl J Med 2018;378(20):1908–1919.
Koné-Paut I, et al. Abstract 1418. Annual Meeting American College of Rheumatology/Association of Rheumatology Health Professionals; 19–24 October 2018, Chicago, IL, US.
Lachmann HJ, et al. N Engl J Med 2009;360(23):2416–2425.
Kuemmerle-Deschner JB, et al. Ann Rheum Dis 2011;70(12):2095–2102.
Brogan PA, et al. Arthritis Rheumatol 2019;71(11):1955–1963.
Kedor C, et al. Ann Rheum Dis 2020;79(8):1090–1097.
Ruperto N, et al. Ann Rheum Dis 2018;77(12):1710–1719.
Ruperto N, et al. N Engl J Med 2012;367(25):2396–2406.
UK | June 2025 | FA-11386252-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.