COMBI-AD efficacy
TAFINLAR® (dabrafenib) in combination with MEKINIST® (trametinib) is indicated in adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.1,2
TAFINLAR in combination with MEKINIST is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.1,2
Common adverse events include:
TAFINLAR + MEKINIST: The most common adverse reactions (incidence ≥20%) for dabrafenib in combination with trametinib were pyrexia, fatigue, nausea, chills, headache, diarrhoea, vomiting, arthralgia and rash1,2
TAFINLAR: The most common adverse reactions (incidence >15%) reported with dabrafenib were hyperkeratosis, headache, pyrexia, arthralgia, fatigue, nausea, papilloma, alopecia, rash and vomiting1
MEKINIST: The most common adverse reactions (incidence ≥20%) for trametinib were rash, diarrhoea, fatigue, oedema peripheral, nausea and dermatitis acneiform2
For more safety information on TAFINLAR and MEKINIST, click here.
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
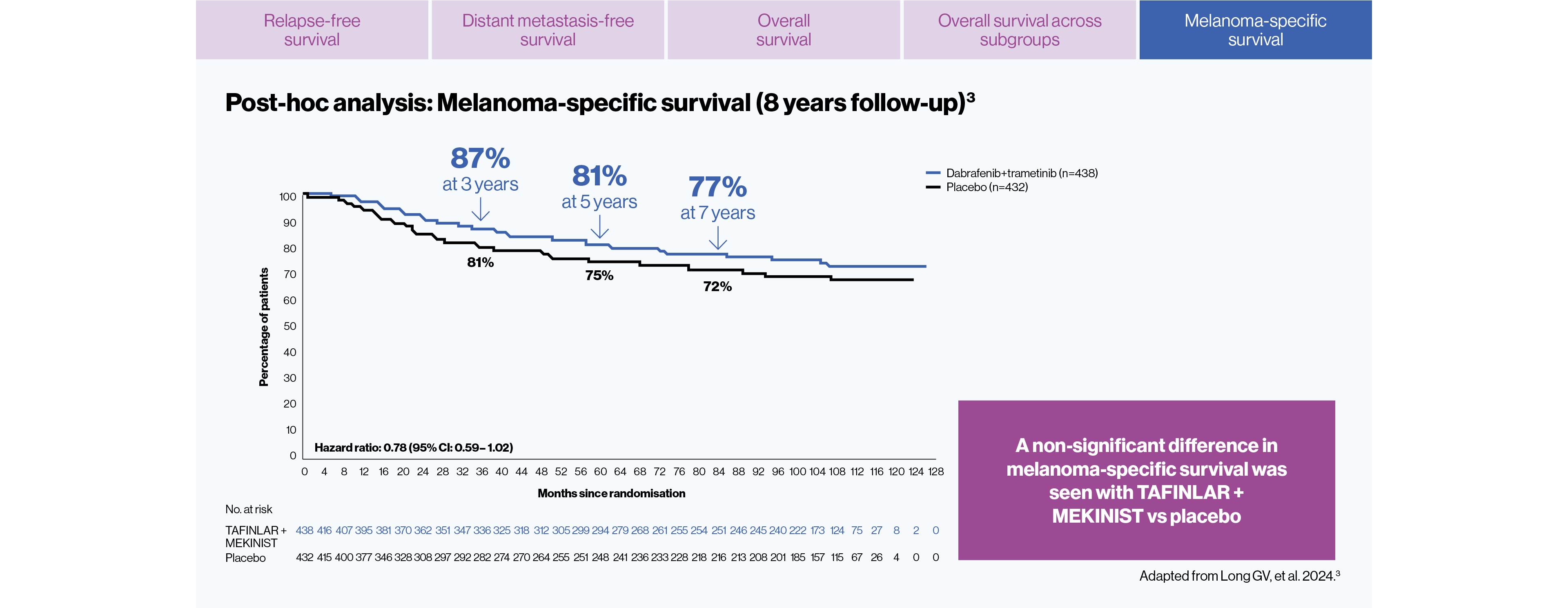
After 8 years of follow up:3
TAFINLAR + MEKINIST showed DURABLE 8-year relapse-free survival vs placebo in adult BRAF V600-positive patients, in a post-hoc analysis3
At a median follow-up of 2.8 years, the estimated 3-year rate of relapse-free survival was 58% in the combination-therapy group and 39% in the placebo group (hazard ratio for relapse or death=0.47; 95% CI: 0.39–0.58, p<0.001).4
Trial design for COMBI-AD
COMBI-AD was a randomised, double-blind, placebo-controlled Phase III trial that compared TAFINLAR + MEKINIST vs placebo in adult patients with Stage III BRAF V600E/K-positive melanoma. The planned duration of treatment was 12 months.3
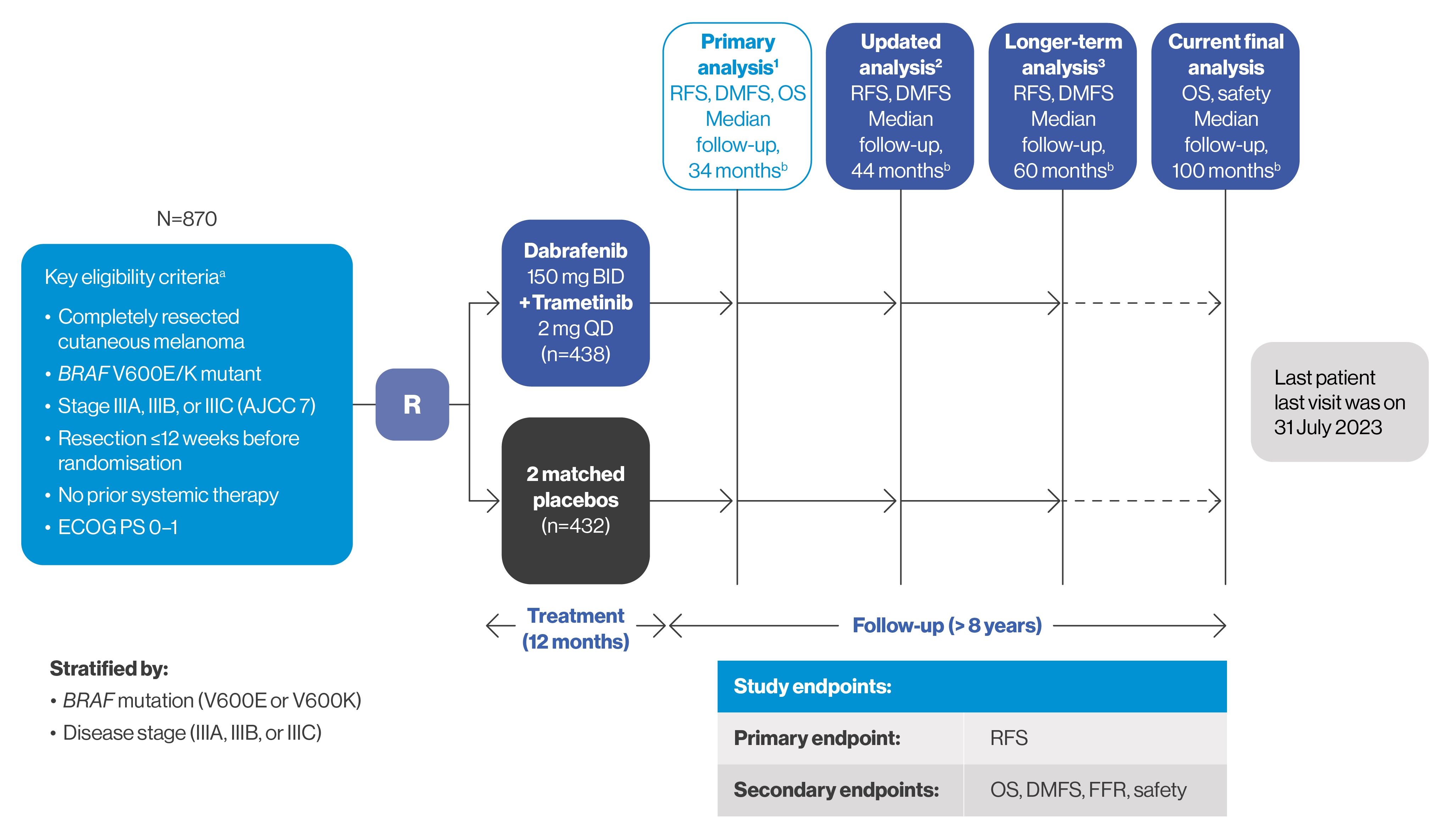
aCOMBI-AD is registered at ClinicalTrials.gov (NCT01682083).
bMedian follow-up shown is for the TAFINLAR plus MEKINIST arm.
Adapted from Long GV, et al. 2024.3
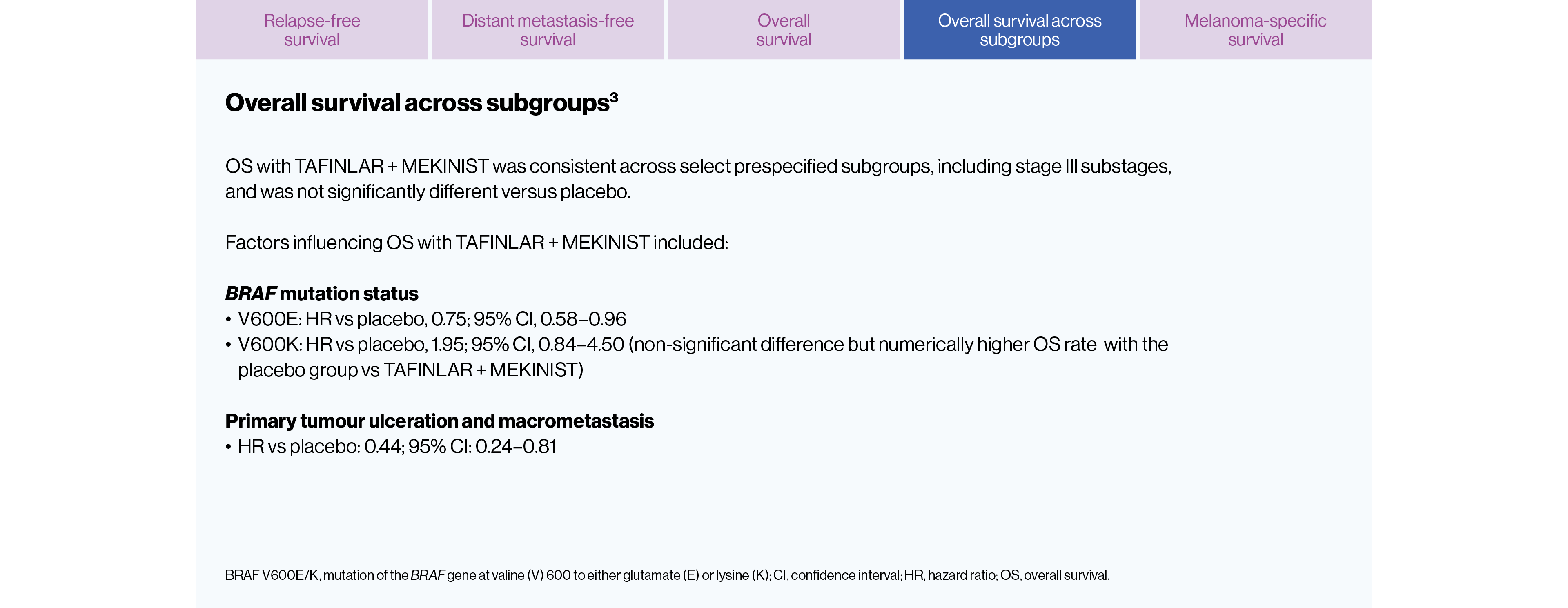
Key clinical data insights
The efficacy and safety of TAFINLAR + MEKINIST for the adjuvant treatment of adult patients with BRAF V600-positive completely resected Stage III melanoma has been studied in a large Phase III trial, COMBI-AD.3
Please visit the COMBI-AD safety page for further information on the TAFINLAR + MEKINIST safety profile in the adjuvant setting.
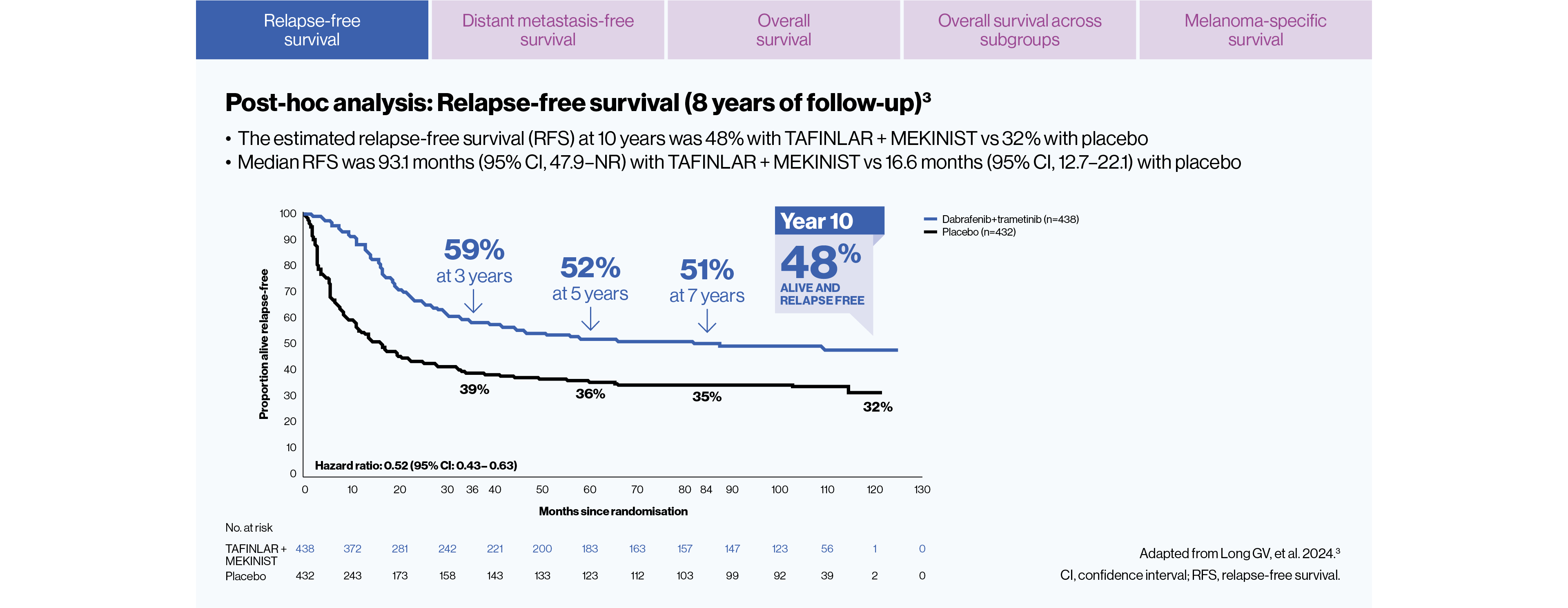
Post-hoc analysis: Durable relapse-free survival (RFS) at 10 years was: 48% (n=438) with TAFINLAR + MEKINIST vs 32% (n=432) with placebo3

Post-hoc analysis: The median RFS was 93.1 months with TAFINLAR + MEKINIST vs 16.6 months with placebo (HR for relapse or death=0.52; 95% CI: 0.43–0.63)3
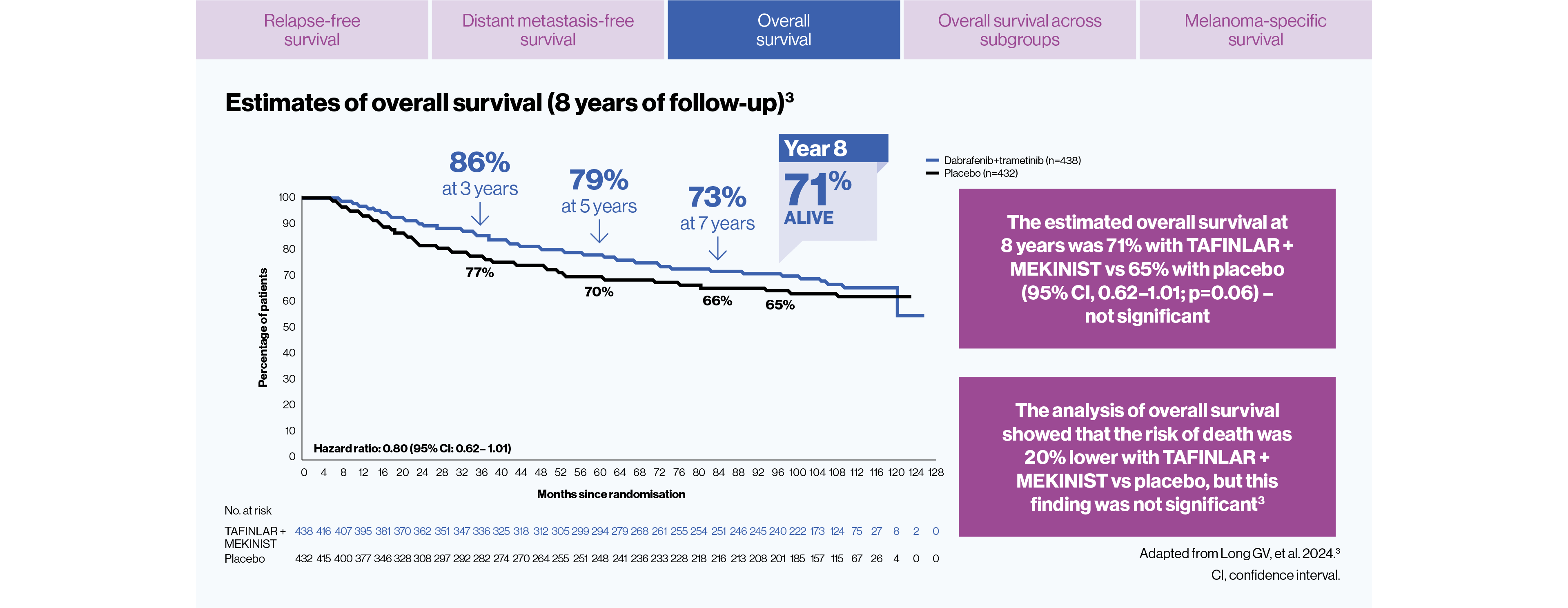
The estimated overall survival at 8 years was 71% with TAFINLAR + MEKINIST vs 65% with placebo. This endpoint did not meet statistical significance (HR for death=0.80; 95% CI:0.62–1.01, p=0.06 by stratified log-rank test)3
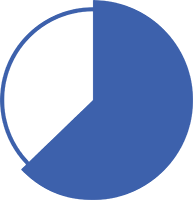
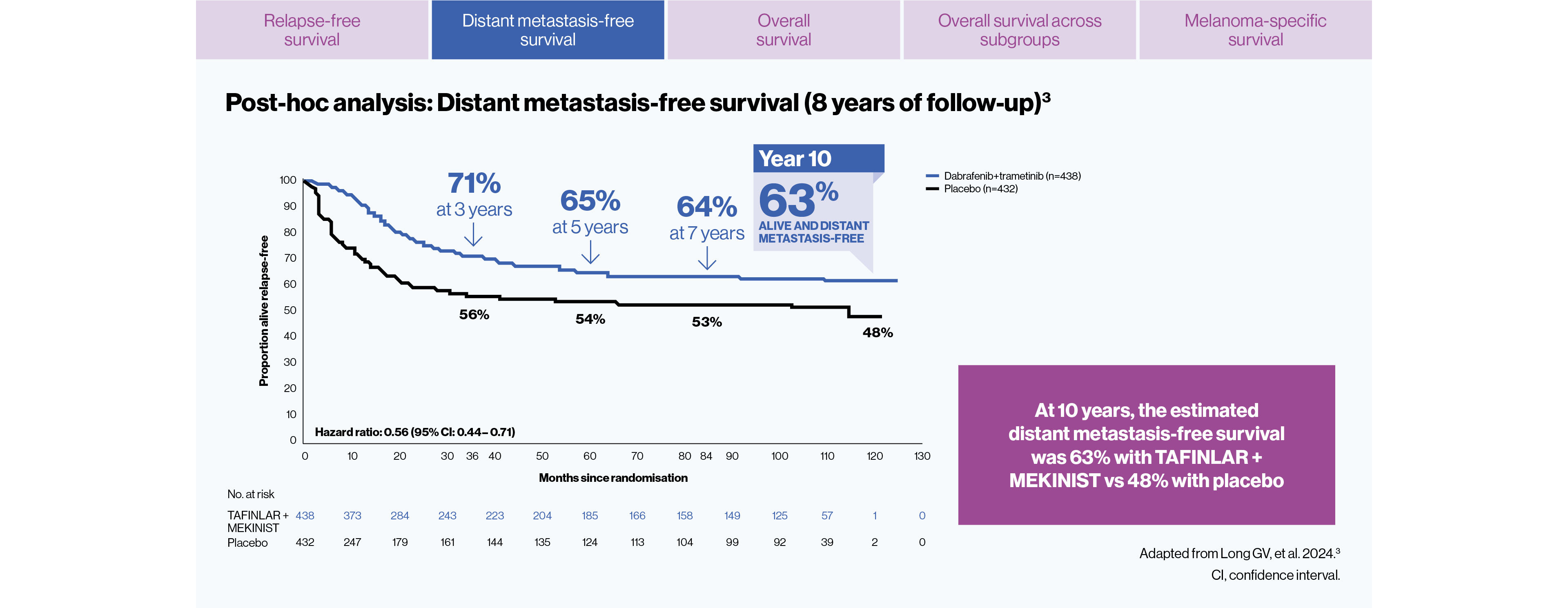
The estimated distant metastasis-free survival at 10 years was 63% (n=438) with TAFINLAR + MEKINIST vs 48% (n=432) with placebo3
AJCC, American Joint Committee on Cancer; BID, twice daily; BRAF V600E/K, mutation of the BRAF gene at valine (V) 600 to either glutamate (E) or lysine (K); CI, confidence interval; DMFS, distant metastasis-free survival; ECOG, Eastern Cooperative Oncology Group; FFR, freedom from relapse; OS, overall survival; QD, once daily; RFS, relapse-free survival; R, randomised; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib). Summary of Product Characteristics.
MEKINIST (trametinib). Summary of Product Characteristics.
Long GV, et al. N Engl J Med 2024;391(18).
Long GV, et al. N Engl J Med 2017;377:1813–1823.
UK | April 2025 | FA-11218657
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.