KISQALI® (ribociclib) and KardiaMobile ECG device
This page is for KISQALI (ribociclib)-prescribing UK healthcare professionals only.
Indications:1
Early breast cancer (eBC)
KISQALI, in combination with an aromatase inhibitor (AI), is indicated for the adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative eBC at high risk of recurrence
In pre perimenopausal women, or in men, the AI should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
Advanced breast cancer (aBC)
KISQALI is indicated for the treatment of women with HR+/HER2– locally advanced or metastatic breast cancer in combination with an AI or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy (ET). In pre- or perimenopausal women, the ET should be combined with a LHRH agonist.
KISQALI is not recommended to be used in combination with tamoxifen.
QT prolongation has been observed with the use of KISQALI. ECG should be assessed before initiating treatment with KISQALI. After initiating treatment, ECG should be repeated at approximately Day 14 of the first cycle, then as clinically indicated. In case of QTcF prolongation during treatment, more frequent ECG monitoring is recommended.1
Treatment with KISQALI should be initiated only in patients with QTcF values less than 450 msec. Based on the observed QT prolongation during treatment, treatment with KISQALI may have to be interrupted, reduced or discontinued.1
Please refer to the Summary of Product Characteristics for the full guidance and information required for monitoring.1
KISQALI and ECG monitoring
The KISQALI & Kardia package deal is organised and funded by Novartis Pharmaceuticals Ltd.
ECG can be performed without the need to remove clothes
Potentially fewer inter-departmental referrals if departments lack ECG equipment
QT measurement obtained within 30 minutes
Follow-up ECGs carried out easily
A solution currently used by over 70 NHS Trusts or Health Boards
Efficiency between hospital departments
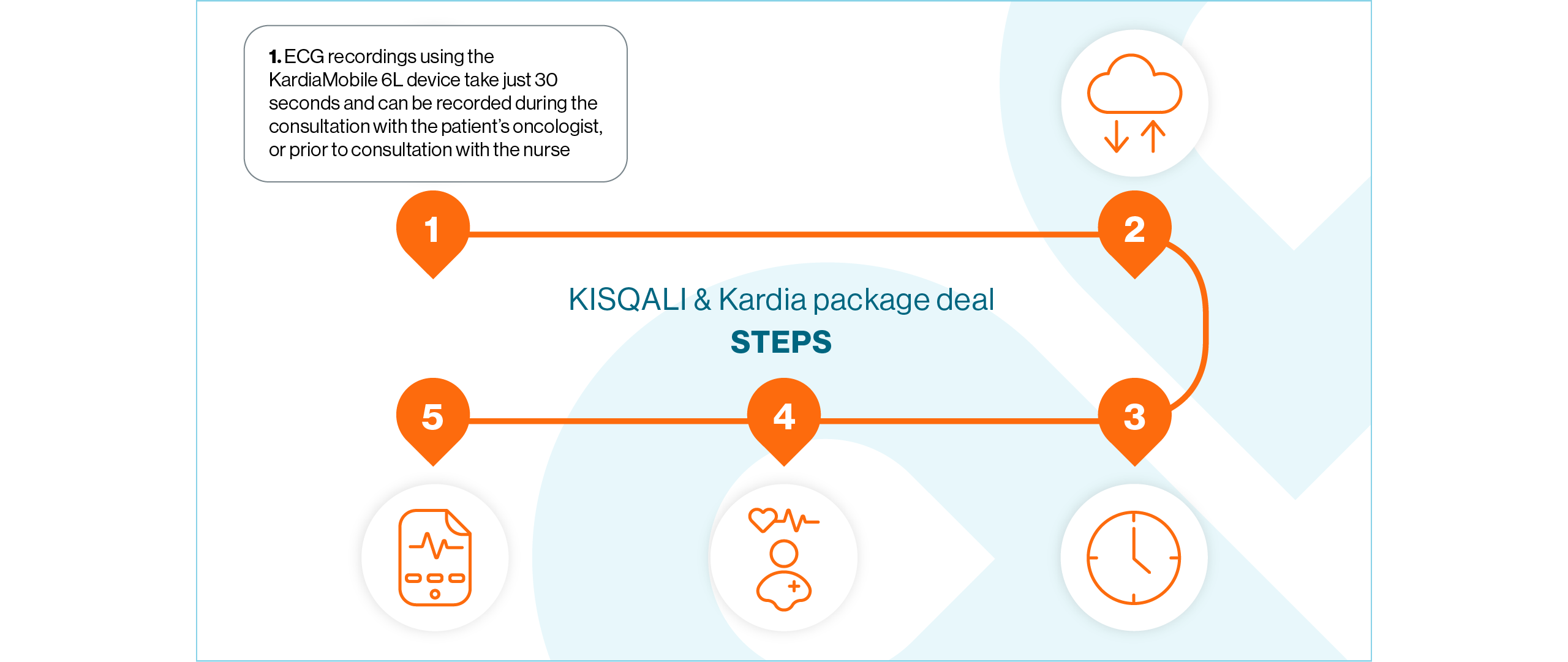
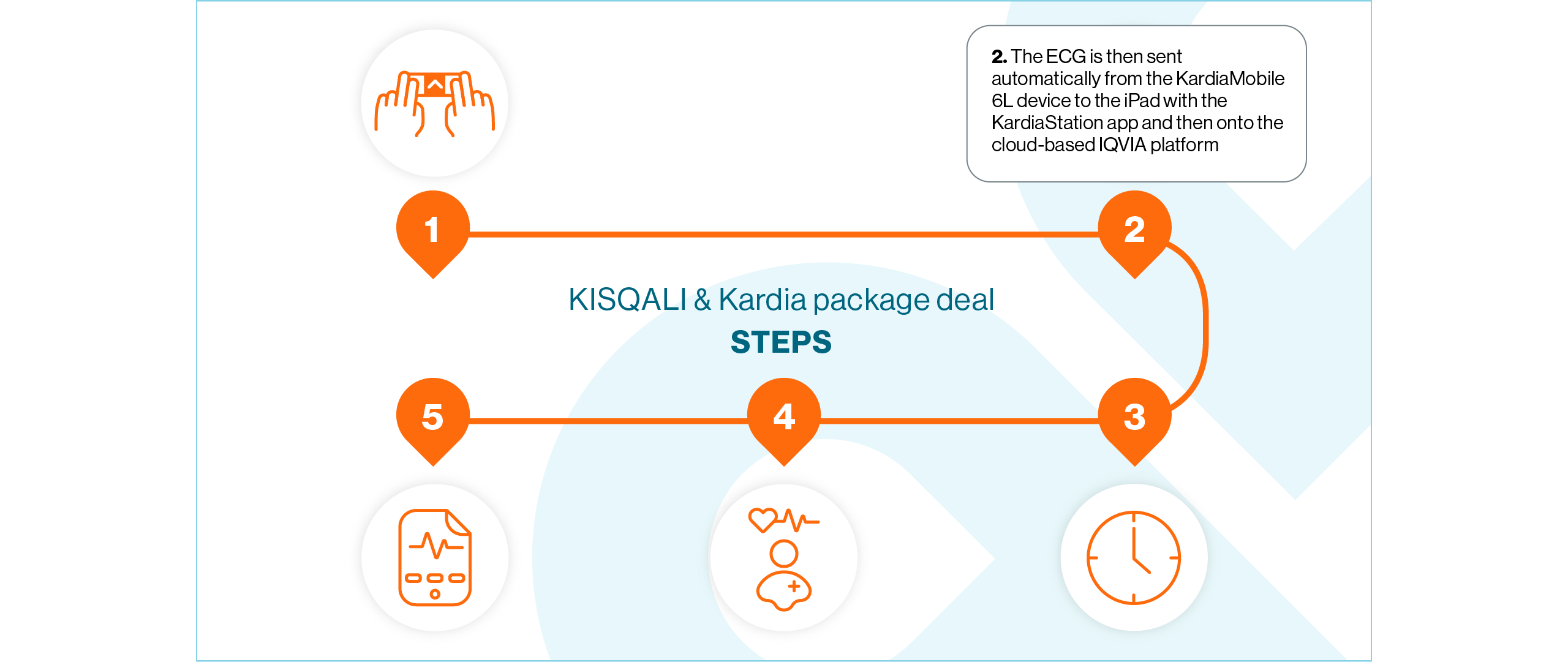
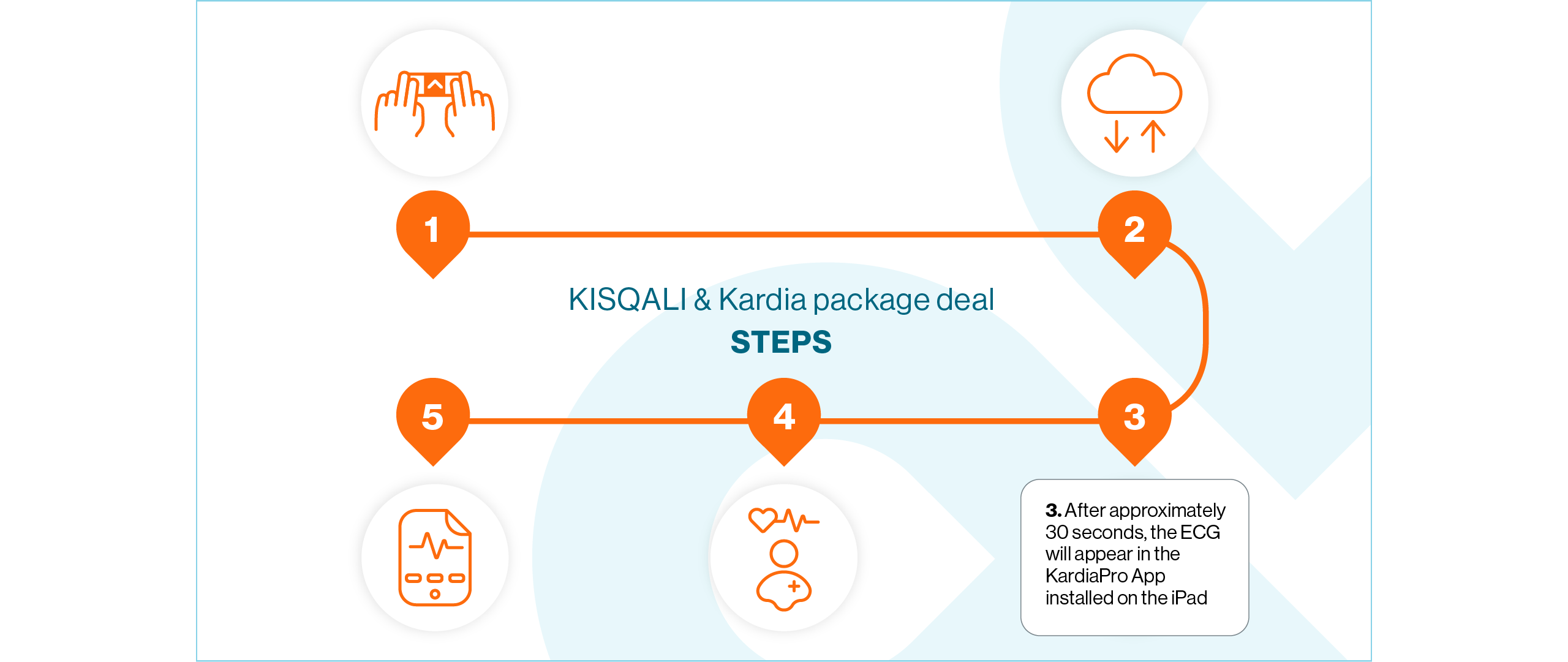
Open the KardiaStation application on the iPad. Once open, click Record ECG
Enter the patient’s medical record number or identifier and press Next
Double-check the correct medical record number or identifier has been entered and confirm the recording by pressing Continue
Select the 6-lead option
When you are ready, give the device to the patient and instruct them to lightly hold it with their thumbs touching the two upper electrodes – no need to squeeze. Ensure the device is in the correct position where the ‘A’ is facing the patient
Whilst continuing to hold their thumbs on the two electrodes, the patient should place the ECG device on the skin of their left leg (knee, or inner ankle). The lower electrode should now be in contact with their skin
Wait for the 30-second countdown to elapse. The ECG recording has now been completed
14 or fewer patients per year – one KardiaMobile device and one iPad
15 to 29 patients per year – two KardiaMobile devices and two iPads
30 or more patients per year – three KardiaMobile devices and three iPads (unless otherwise requested by the NHS Trust Health Board and agreed in writing by Novartis)
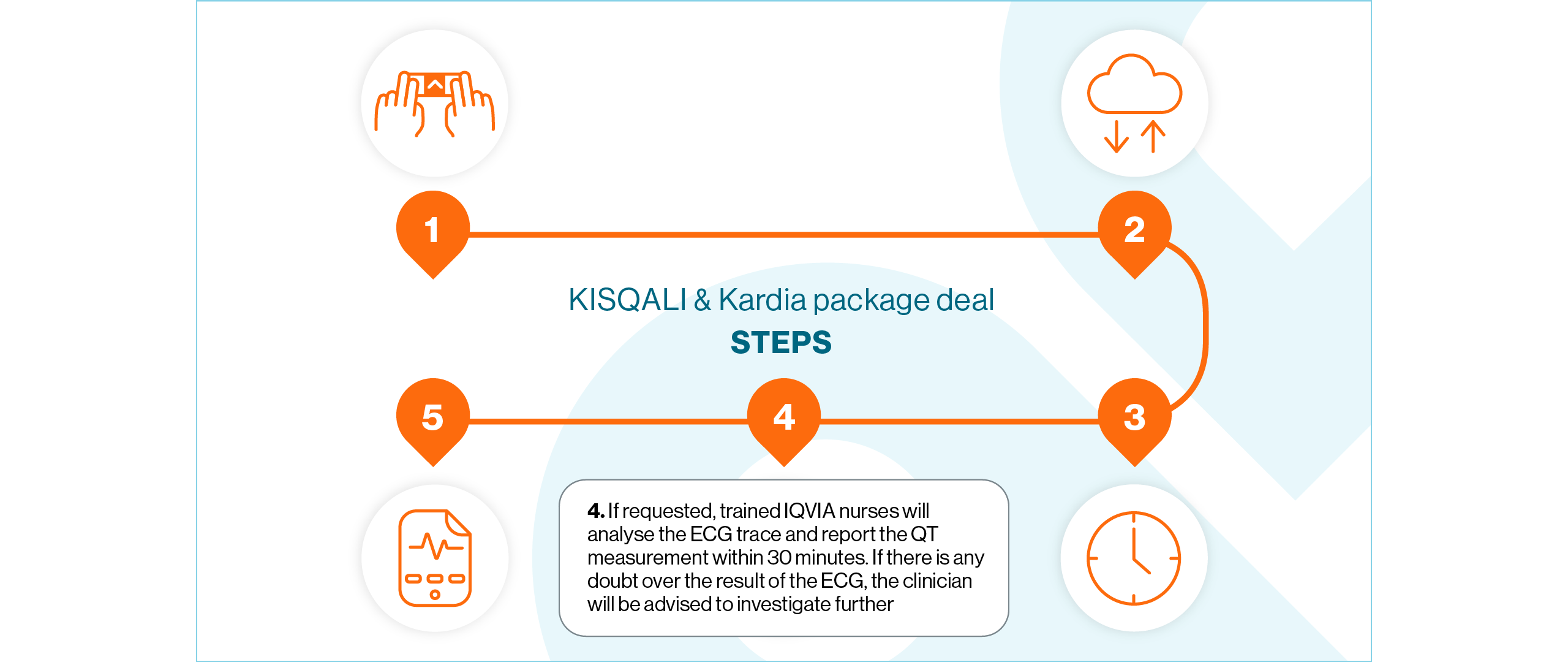
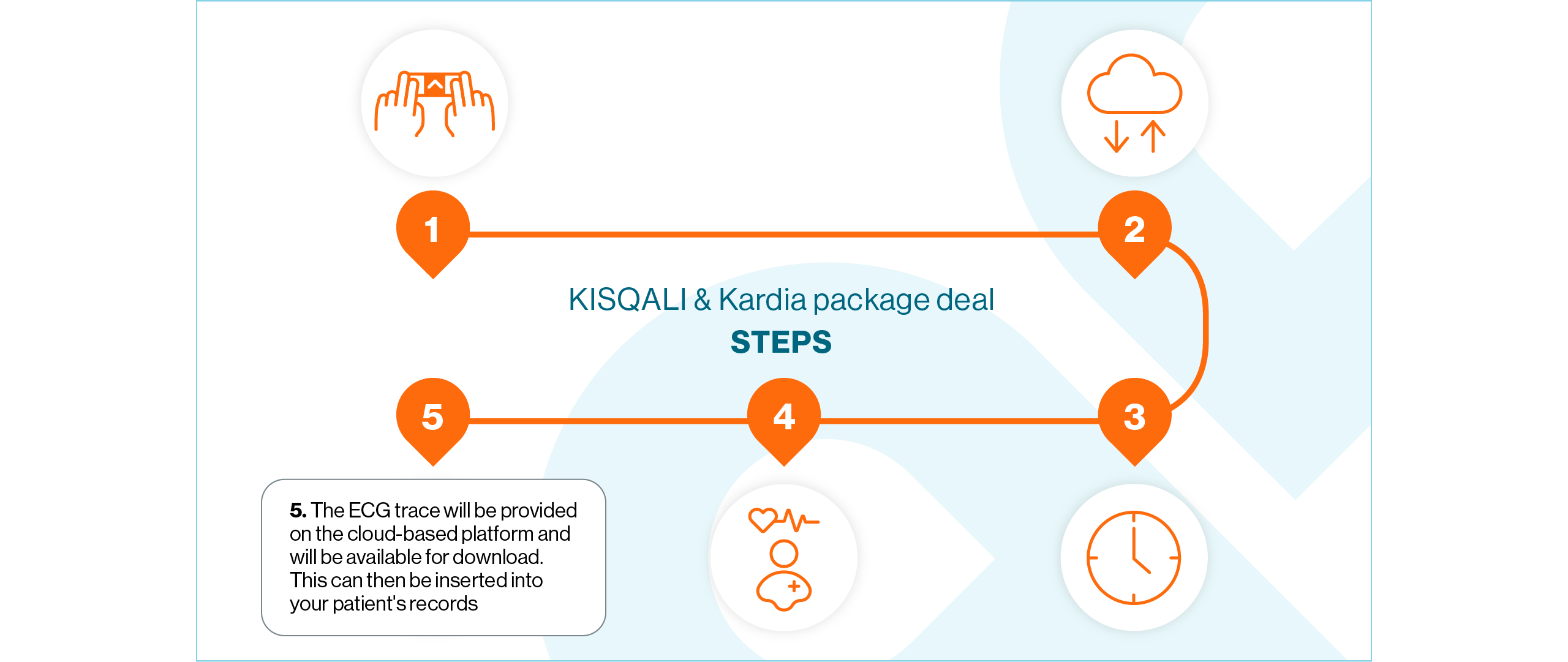
The KISQALI & Kardia package deal gives your patients access to technology that will supplement oncology clinical practice
What does the package deal offer?

This package deal is for an initial period of 12 months, with the option to extend. Novartis have elected to work with IQVIA, who will provide the package deal to NHS Trusts or Health Boards on behalf of Novartis.
Novartis will provide this package to NHS Trusts or Health Boards in accordance with Clause 19.1 of the ABPI Code of Practice 2024, as part of the purchase price of KISQALI. For the avoidance of doubt, there is no obligation for NHS Trusts or Health Boards to whom the package deal is offered to purchase any specific minimum volume of KISQALI or any other Novartis product.
How does it work?
View the steps below to find out how the KISQALI & Kardia package deal works:






What are the benefits of using the KardiaMobile ECG device?

BENEFITS FOR PATIENTS

BENEFITS FOR HEALTHCARE PROFESSIONALS

BENEFITS FOR THE NHS
Recording a 6L ECG2
Please refer to the Instructions for Use for KardiaMobile 6L for further information.2
Q: Why is this offered?
A: QT prolongation has been observed with the use of KISQALI. ECG should be assessed before initiating treatment with KISQALI in all patients. Treatment with KISQALI should be initiated only in patients with QTcF values less than 450 msec. After initiating treatment, ECG should be repeated at approximately day 14 of the first cycle, then as clinically indicated.1 In case of QTcF prolongation during treatment, more frequent ECG monitoring is recommended in patients.1 Based on the observed QT prolongation during treatment, treatment with KISQALI may have to be interrupted, reduced or discontinued as described in the SmPC. Please refer to the SmPC for further information.1
Q: Once the programme is in place, how do I access support when required?
A: Upon setting up the programme in your NHS Trust or Health Board, IQVIA will provide you with a helpline number.
Q: Is the KardiaMobile battery operated, or can it be charged?
A: The KardiaMobile is powered by a CR2016 battery and it is recommended the battery is changed annually.
Q: Can the ECG trace be uploaded to patient records?
A: The ECG trace can be downloaded as a PDF and then uploaded to patient records by the HCP. Before the programme is set up at the NHS Trust or Health Board, you will receive training on the use of the device.
Q: How many devices will I receive?
A: The number of devices you will receive will be based upon the approximate number of new diagnoses of HR+/HER2– eBC or aBC your centre receives each year. You will be expected to provide this detail to IQVIA during the contracting process. (Please note, there is a limit of 3 devices per centre)
Q: How will the KardiaMobile and iPad be maintained?
A: The KardiaMobile and the iPad tablet will be covered by a manufacturer’s warranty for 12 months, following which the cost of reasonable repair will be charged to Novartis Ltd., provided that these do not result from abnormal or incorrect use or damage by the Site. If damaged, IQVIA will aim to replace it within 48 hours.
Q: Can we use the KardiaMobile device to carry out an ECG for other treatments or conditions?
A: No, this package deal is intended only for use with patients where there is an intent to prescribe or where patients have been prescribed KISQALI.
Q: Is there a cost to the NHS for this service?
A: There is no cost to the NHS. This package deal is offered by Novartis Ltd. and is a commercial offering linked to the purchase price of KISQALI. Novartis Ltd. will provide NHS Trusts or Health Boards in accordance with Clause 19.1 of the ABPI Code of Practice 2024, as part of the purchase price of KISQALI. For the avoidance of doubt, there is no obligation for NHS Trusts or Health boards to whom the package deal is offered to purchase any specific minimum volume of KISQALI or any other Novartis product.
Click here for additional resources for both you and your patients
The KISQALI & Kardia package deal is organised and funded by Novartis Pharmaceuticals Ltd.
6L, 6-lead; aBC, advanced breast cancer; ABPI, Association of the British Pharmaceutical Industry; eBC, early breast cancer; ECG, electrocardiogram; FAQ, frequently asked question; HCP, healthcare professional; HER2–, human epidermal growth receptor 2-negative; HR+, hormone receptor-positive; LHRH, luteinising hormone-releasing hormone; NHS, National Health Service; QTcF, corrected QT interval by Fridericia's formula; SmPC, summary of product characteristics.
References
KISQALI® (ribociclib) Summary of Product Characteristics.
AliveCor® KardiaMobile 6L. Instructions for Use. 2022.
UK | July 2025 | 442300
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.