LUXTURNA®
Indications
Luxturna is indicated for the treatment of adult and pediatric patients with inherited retinal dystrophy caused by confirmed biallelic RPE65 mutations and who have sufficient viable retinal cells as determined by the treating physician(s).
Disease-causing biallelic RPE65 mutations should be confirmed by an accredited laboratory using validated assay methods.
Novartis Risk Management Plan (RMP) Educational Materials for Patients
Image
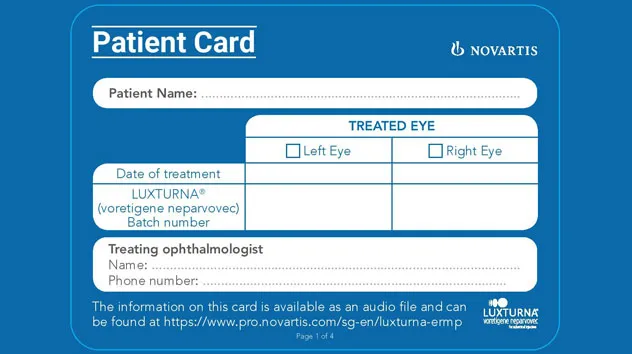

15 Jan 2026
4 mins 8 secs
 AUDIO
AUDIO
Image


15 Jan 2026
17 mins 2 secs
 AUDIO
AUDIO


