

KISQALI® (ribociclib): Quality of Life (QoL)
Maintaining quality of life is important to patients with metastatic breast cancer.2
Data from a matching-adjusted indirect comparison offers insights into how KISQALI and abemaciclib, both combined with AI, impact symptom-related QoL for HR+ HER2- patients.3
The anchored matching-adjusted indirect comparison compared patient-report QoL outcomes from MONALESSA-2 (KISQALI + AI) and MONARCH 3 (abemaciclib + AI). Individual patient data from MONALEESA-2 was matched and balanced against published data from MONARCH 3 and time to sustained deterioration (TTSD) in EORTC-QLQ-C30 and BR-23 questionnaire scores were compared.3
KISQALI + AI was associated with better symptom-related QoL vs abemaciclib + AI†3
†Time to sustained deterioration in symptom, 1L postmenopausal women with HR+ HER2- MBCa3
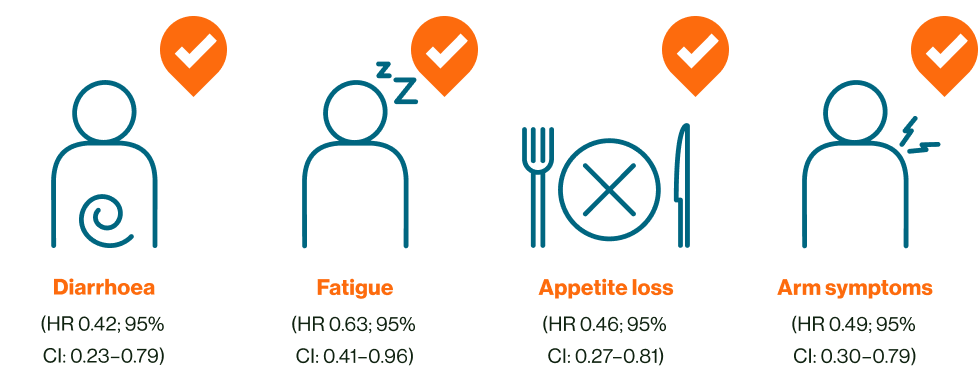
How can reduction in these AEs benefit the QoL of your patients?
aMatching-adjusted indirect comparisons should be interpreted with caution. There are no head-to-head trials between KISQALI and abemaciclib. This analysis may be confounded by unreported factors as only published characteristics for MONARCH 3 were controlled for. Additionally, extreme weightings required for some patients during matching adjustment may have led to low statistical power for detecting differences between treatments.3
The most common adverse events in KISQALI treated patients (reported at a frequency ≥20%) across EBC and MBC were neutropenia, nausea, infections, fatigue, diarrhoea, alopecia, leukopenia, constipation, headache, cough, anaemia, abnormal liver function tests and abdominal pain. Additional common ARs in EBC included asthenia and pyrexia, while those in MBC included vomiting, back pain, anaemia, rash and decreased appetite1
1L, first-line; AE, adverse event; AI, aromatase inhibitor; CI, confidence interval; HR, hazard ratio; MAIC, matching-adjusted indirect comparison; MBC, metastatic breast cancer; QoL, quality of life.
References:
KISQALI Australian approved Product Information
Mertz S, et al. Breast. 2022; 65: 84–90
Rugo HS, et al. Ther Adv Med Oncol. 2023;15:1–10
PBS Information: Authority Required. For the treatment of advanced/metastatic breast cancer and early breast cancer at high risk of recurrence meeting PBS criteria. Refer to the PBS Schedule for full Authority information.
|
For KISQALI (ribociclib) prescribing information, please click here.
For KISQALI (ribociclib) PBS status, please click here.
Ward7 NOKI36873M. AU-29121. August 2025.