

TAFINLAR + MEKINIST is the first and only† targeted treatment for low-grade paediatric glioma with BRAF V600E mutations who require systemic therapy1,2
†As of July 2025
Surgery aimed at maximal safe surgical resection followed by first-line chemotherapy is the current first-line treatment for most paediatric patients with LGG. The use of radiotherapy is typically reserved for older patients (>3 years) or those refractory to medical therapy.3
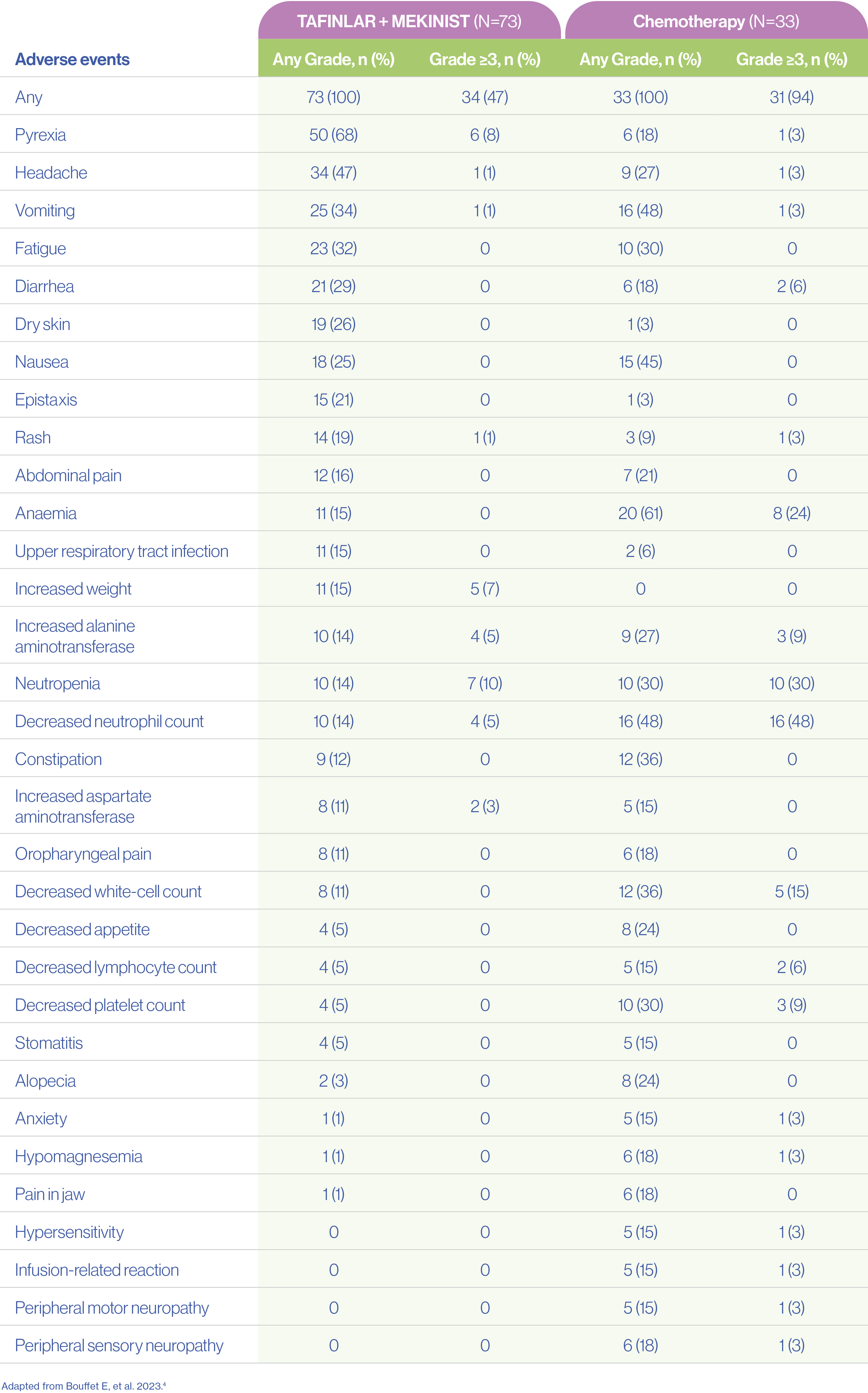
Paediatric patients treated with TAFINLAR + MEKINIST had fewer Grade ≥3 adverse events compared with chemotherapy (47% vs 94%)4
Adverse events (safety population)
TAFINLAR + MEKINIST demonstrated significantly higher ORR compared with chemotherapy in paediatric patients with low-grade glioma†4
†Patients treated with TAFINLAR + MEKINIST had an ORR of 47% compared with 11% for those treated with chemotherapy (95% CI: 1.7–11.2; p<0.001)4
Primary endpoint: Objective response rate4
TAFINLAR + MEKINIST significantly improved PFS compared with chemotherapy in paediatric patients with low-grade glioma†4
Progression-free survival4
TAFINLAR and MEKINIST PBS Information: Authority Required. Please refer to PBS Schedule for full authority information for products.
Footnotes and References
Abbreviations: CI, confidence interval; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PBS, Pharmaceutical Benefits Scheme; PFS, progression-free survival.
References: 1. TAFINLAR (dabrafenib) Australian approved product information. 2. MEKINIST (trametinib) Australian approved product information. 3. Sait SF, et al. Curr Neurol Neurosci Rep. 2023;23(4):185–199. 4. Bouffet E, et al. N Engl J Med. 2023;389:1108–1120
