

KISQALI®(ribociclib): Elderly patients
The MONALEESA-2 study demonstrated that KISQALI + NSAI significantly improves PFS†2 and OS‡3 in patients with HR+ HER2- MBC versus NSAI alone.
†mPFS KISQALI + NSAI 25.3 months vs placebo + NSAI 16.0 months (HR 0.568, 95% CI: 0.457–0.704; p=9.63 x 10-8)2
‡mOS KISQALI + NSAI 63.9 months vs placebo + NSAI 51.4 months; (HR 0.76, 95% CI: 0.63–0.93; two-sided p=0.008), secondary endpoint3
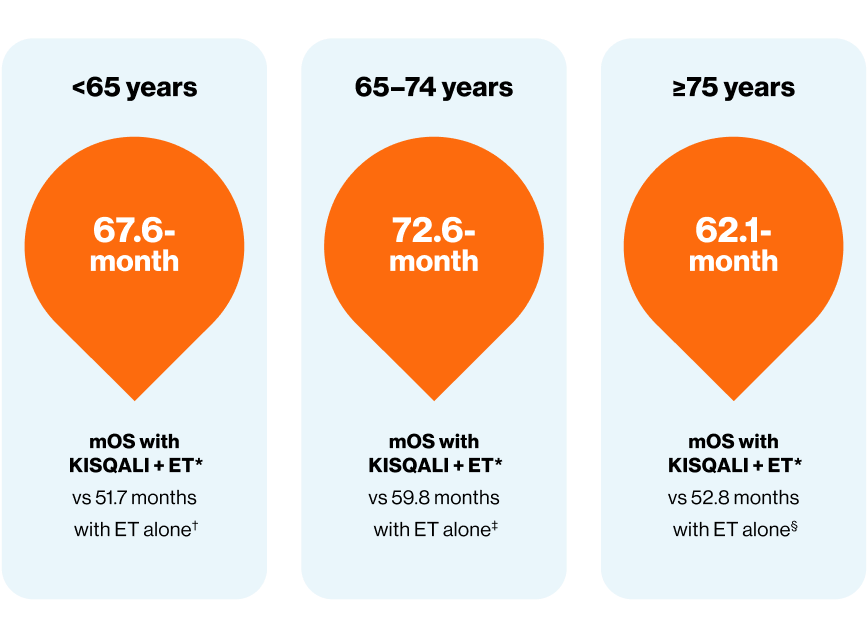
In an exploratory pooled analysis, 1L KISQALI + ET patients demonstrated consistent mOS across age groups*4
*Exploratory analysis of pooled MONALEESA data. mOS in KISQALI + ET patients across age groups: <65 years 67.6 months, 65–74 years 72.6 months, and ≥75 years 62.1 months, no p-values reported4
Exploratory MONALEESA pooled analysis4
Exploratory analysis showed QoL was preserved or improved across all age groups versus ET alone^4
^Exploratory analysis of pooled MONALEESA data. mTTD in GHS across age groups: <65 years HR 0.61 (95% CI: 0.47–0.80) log-rank p=1.70x10-4; 65–74 years HR 0.90 (95% CI: 0.57–1.42) log-rank p=0.321, not significant; ≥75 years HR 1.26 (95% CI: 0.57–2.80) log-rank p=0.723, not significant4
The most common adverse events in KISQALI treated patients (reported at a frequency ≥20%) across EBC and MBC were neutropenia, nausea, infections, fatigue, diarrhoea, alopecia, leukopenia, constipation, headache, cough, anaemia, abnormal liver function tests and abdominal pain. Additional common adverse events in EBC included asthenia and pyrexia, while those in MBC included vomiting, back pain, anaemia, rash and decreased appetite1
1L, first-line; AI, aromatase inhibitor; CI, confidence interval; combo CT, combination chemotherapy; ET, endocrine therapy; GHS, global health score; HER2-, human epidermal growth factor receptor 2 negative; HR, hazard ratio; HR+ hormone receptor positive; MBC, metastatic breast cancer; mPFS, median progression-free survival; mTTD, median time to deterioration; OS, overall survival; PFS, progression-free survival; QoL, quality of life.
References:
KISQALI Australian approved Product Information.
Hortobagyi GN, et al. Ann Oncol. 2018; 29(7): 1541–1547.
Hortobagyi GN, et al. N Engl J Med. 2022; 386(10): 942–950
Hart LL, et al. Eur J Cancer. 2025; 217: 115225
PBS Information: Authority Required. For the treatment of advanced/metastatic breast cancer and early breast cancer at high risk of recurrence meeting PBS criteria. Refer to the PBS Schedule for full Authority information.
|
For KISQALI (ribociclib) prescribing information, please click here.
For KISQALI (ribociclib) PBS status, please click here.
Ward7 NOKI36871M. AU-29119. August 2025.
