

KISQALII® (ribociclib): Right Choice
The RIGHT Choice study demonstrated significant improvement in mPFS for patients with HR+ HER2- MBC treated with KISQALI in combination with AI compared to patients receiving combination chemotherapy. The study reported a mPFS of 21.8 months with KISQALI plus AI versus 12.8 months with combo CT (HR 0.61 95% CI: 0.43–0.87; p=0.003).2
Significant mPFS benefit with KISQALI vs combo CT in patients with aggressive disease — RIGHT Choice†2
†KISQALI + AI mPFS 21.8 months vs 12.8 months for combo CT; HR 0.61 (95% CI: 0.43–0.87); p=0.003a2

How would this mPFS increase be an improvement over what you expect from your existing treatments for aggressive disease?
In an exploratory sub-analysis of RIGHT Choice, time to deterioration in QoL was longer for 1L KISQALI + ET vs combo CT in patients with aggressive disease‡3
‡Abstract data. mTTD based on the composite end point for overall health status score KISQALI + ET 16.8 months vs 10.6 months for combo CT (HR 0.63 95% CI: 0.44–0.9; no p-value reported)b2,3
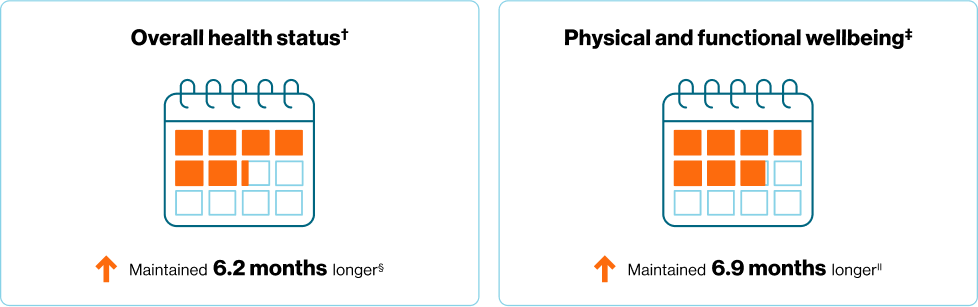
What would it mean to you to be able to maintain your patients' QoL for 6 extra months?
aRIGHT Choice primary endpoint; mPFS KISQALI + AI 21.8 months vs combo CT 12.8 months HR: 0.61 (95% CI: 0.43–0.87); p=0.0032
bExploratory analysis of RIGHT Choice. Aggressive disease features included symptomatic visceral metastases, rapid disease progression or impending visceral compromise, or markedly symptomatic non-visceral disease2,3
The most common adverse events in KISQALI treated patients (reported at a frequency ≥20%) across EBC and MBC were neutropenia, nausea, infections, fatigue, diarrhoea, alopecia, leukopenia, constipation, headache, cough, anaemia, abnormal liver function tests and abdominal pain. Additional common adverse events in EBC included asthenia and pyrexia, while those in MBC included vomiting, back pain, anaemia, rash and decreased appetite1
1L, first-line; AI, aromatase inhibitor; CI, confidence interval; combo CT, combination chemotherapy; EBC, early breast cancer; ET, endocrine therapy; HR, hazard ratio; MBC, metastatic breast cancer; mPFS, median progression-free survival; mTTD, median time to deterioration; QoL, quality of life.
PBS Information: Authority Required. For the treatment of advanced/metastatic breast cancer and early breast cancer at high risk of recurrence meeting PBS criteria. Refer to the PBS Schedule for full Authority information.
|
For KISQALI (ribociclib) prescribing information, please click here.
For KISQALI (ribociclib) PBS status, please click here.
Ward7 NOKI36879M. AU-29125. August 2025.
References
KISQALI Australian approved Product Information.
Lu Y-S, et al. J Clin ONcol. 2024; 42(23): 2812–2821.
Eralp Y, et al. Poster presentation 456P. Presented at European Society for Medical Oncology 2023, 20–24 October, Madrid, Spain.
