

KISQALI® (ribociclib): Overall Survival MAIC Study
Living longer is the ultimate goal for patients with metastatic breast cancer.2
Findings from a matching-adjusted indirect comparison (MAIC) study have highlighted the differences in overall survival KISQALI and palbociclib in HR+ HER2- MBC patients.3
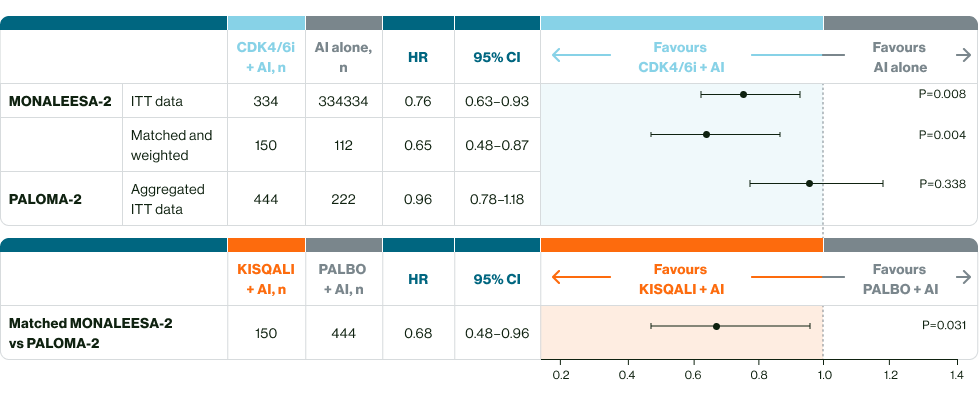
Significantly greater OS benefit with 1L KISQALI + AI vs palbociclib + AI†3
†Observed mOS benefit based on indirect analysis in 1L patients with HR+ HER2- MBC (HR 0.68; p=0.031)a3
MAIC study design3
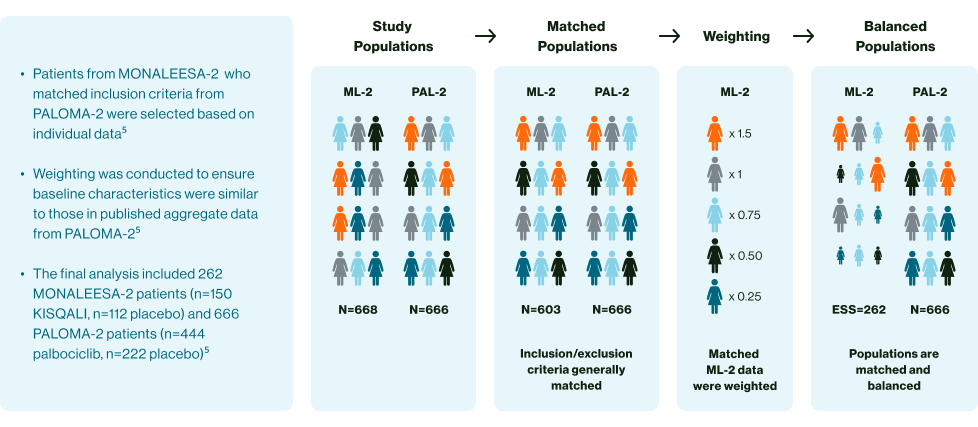
aMatching-adjusted indirect comparisons should be interpreted with caution. Currently there are no head-to-head trials between KISQALI and palbociclib. This anchored MAIC study utilised patient data from the MONALEESA-2 trial for ribociclib and the PALOMA-2 trial for palbociclib to indirectly compare the effectiveness of ribociclib and palbociclib, using trial data and adjusting for known differences, however limitations include potential bias from missing data or unreported data3
The most common adverse events in KISQALI treated patients (reported at a frequency ≥20%) across EBC and MBC were neutropenia, nausea, infections, fatigue, diarrhoea, alopecia, leukopenia, constipation, headache, cough, anaemia, abnormal liver function tests and abdominal pain. Additional common adverse events in EBC included asthenia and pyrexia, while those in MBC included vomiting, back pain, anaemia, rash and decreased appetite1
AI, aromatase inhibitor; CI, confidence interval; ESS, effective sample size; ET, endocrine therapy; HER2-, human epidermal growth factor receptor 2 negative; HR, hazard ratio; HR+, hormone receptor positive; LHRH, luteinising hormone-releasing hormone agonist; MAIC, matching-adjusted indirect comparison; MBC, metastatic breast cancer; ML-2,MONALEESA-2; mOS, median overall survival; OS, overall survival; PAL-2, PALOMA-2; PFS, progression-free survival3
References:
KISQALI Australian approved Product Information.
Mertz S, et al. Breast. 2022; 65: 84–90.
Jhaveri K, et al. Ther Adv Med Oncol. 2023; 15: 1–11.
PBS Information: Authority Required. For the treatment of advanced/metastatic breast cancer and early breast cancer at high risk of recurrence meeting PBS criteria. Refer to the PBS Schedule for full Authority information.
|
For KISQALI (ribociclib) prescribing information, please click here.
For KISQALI (ribociclib) PBS status, please click here.
Ward7 NOKI36876M. AU-29123. August 2025.
Adverse events should be reported.
Adverse events and product complaints should also be reported to Novartis.
For Medical Enquiries, Information Services, Adverse Events and Product Complaints please contact: 1800 671 203 or [email protected]
Colleagues are available from 9:00 to 17:00 from Monday to Friday.
