

KISQALI® (ribociclib): mOS in Visceral Metastases
KISQALI has demonstrated OS improvements across a range of HR+ HER2- MBC patients.2–5
KISQALI has demonstrated consistent mOS benefit across 3 phase III trials†2–4
†HR+ HER2- MBC patients versus placebo. M2: KISQALI mOS 63.9 months vs placebo 51.4 months; P=0.008a2 M7: KISQALI mOS not reached vs placebo 40.9 months; P=0.00973b3 M3: KISQALI mOS not reached vs placebo 40.0 months; P=0.00455c4
"Overall survival is the hardest endpoint to achieve in clinical trials, and in many respects, perhaps the most important...we're trying to improve the survival of the patient, not just the progression-free survival..."
Dennis Slamon, MD, PhD
University of California, USA
Pooled analysis by Yardley et al. explored the impact of 1L KISQALI + ET in patients with visceral metastases.5
Exploratory pooled analysis of 1L patients with visceral disease enrolled in MONALESSA-2, -3 and -7‡2–5
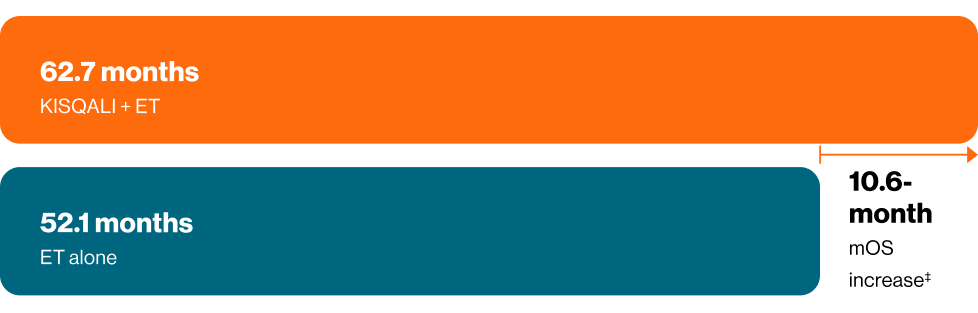
‡Abstract exploratory data, caution should be taken when interpreting results. KISQALI + ET mOS 62.7 vs 52.1 months for ET alone in 1L HR+ HER2- MBC patients with visceral metastases (HR 0.79 CI: 0.65–0.97; p=0.023)d2–5
mOS in 1L patients with visceral metastases — MONALEESA pooled analysis
aMONALEESA-2 secondary endpoint ITT population; mOS KISQALI + NSAI 63.9 months (95% CI: 52.4–71.0) vs placebo + NSAI 51.4 months (95% CI: 47.2–59.7); HR: 0.76 (95% CI: 0.63–0.93), P=0.008; Primary endpoint (mPFS) met2
bMONALEESA-7 secondary endpoint ITT population; mOS KISQALI + ET not reached vs placebo + ET 40.9 months (95% CI: 37.8–NR); HR: 0.71 (95% CI: 0.54–0.95), P=0.00973; Primary endpoint (mPFS) met3
cMONALEESA-3 secondary endpoint ITT population; mOS KISQALI + fulvestrant not reached vs placebo + fulvestrant 40.0 months (95% CI: 37.0–NR); HR: 0.72 (95% CI: 0.57–0.92), P=0.00455; Primary endpoint (mPFS) met4
dAbstract exploratory data, caution should be taken when interpreting results, Pooled analysis of 1,124 patients with visceral metastases (including liver or ≥3 disease sites) from MONALEESA studies. mOS 1L patients KISQALI + ET (n=395) 62.7 months vs 52.1 months for placebo + ET (n=319) (HR 0.79; 95% CI: 0.65–0.97; p=0.23)2–5
The most common adverse events in KISQALI treated patients (reported at a frequency ≥20%) across EBC and MBC were neutropenia, nausea, infections, fatigue, diarrhoea, alopecia, leukopenia, constipation, headache, cough, anaemia, abnormal liver function tests and abdominal pain. Additional common adverse events in EBC included asthenia and pyrexia, while those in MBC included vomiting, back pain, anaemia, rash and decreased appetite1
1L, first-line; CI, confidence interval; EBC, early breast cancer; ET, endocrine therapy; GHS, global health score; HER2-, human epidermal growth factor receptor 2 negative; HR, hazard ratio; HR+ hormone receptor positive; MBC, metastatic breast cancer; mOS, median overall survival; mPFS, median progression-free survival; OS, overall survival; QoL, quality of life.
References:
KISQALI Australian approved Product Information.
Hortobagyi GN, et al. N Engl J Med. 2022; 386(10): 942–950.
Im SA, et al. N Engl J Med. 2019; 381(4): 307–316.
Slamon DJ, et al. N Engl J Med. 2020; 382(6): 514–524.
Yardley DA, et al. Abstract 205P. Presented at ESMO Congress Paris, France 9–13 September 2022.
PBS Information: Authority Required. For the treatment of advanced/metastatic breast cancer and early breast cancer at high risk of recurrence meeting PBS criteria. Refer to the PBS Schedule for full Authority information.
|
For KISQALI (ribociclib) prescribing information, please click here.
For KISQALI (ribociclib) PBS status, please click here.
Ward7 NOKI36881M. AU-29127. August 2025.
