
Cosentyx UnoReady pen self-injection video.
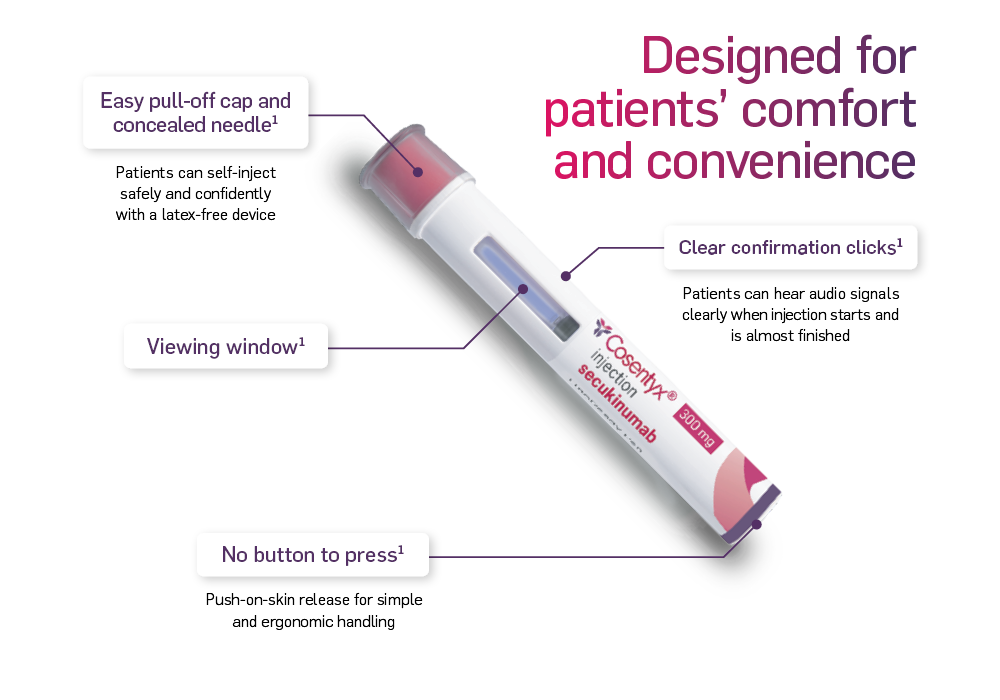
* For patients with Hidradenitis Suppurativa, a maintenance dose of 300 mg every 2 weeks may be prescribed.
† In the MATURE trial, a randomised, double-blind, placebo-controlled, multicenter 52-week study, the efficacy, safety and pharmacokinetics of Cosentyx as administered by the 300 mg pen were evaluated in patients with moderate to severe plaque psoriasis. All patients (n=37) in the active 300 mg pen arm reported being satisfied or very satisfied with the device at Week 28 in a self-injection assessment questionnaire.2
‡ In the same clinical study, of the 854 active injections administered in the 300 mg pen arm, only 1 injection site reaction was reported through Week 48.2
Adverse events: Very common (≥10%) nasopharyngitis. Common (≥1 to <10%): upper respiratory tract infection, rhinitis, pharyngitis, oral herpes, diarrhoea,
urticaria, dermatitis (including eczema), rhinorrhoea, headache, nausea, hypercholesterolemia
References: 1. Cosentyx Approved Product Information. March 2025. 2. Sigurgeirsson B et al. Dermatol Ther. 2022;35(3):e15285. doi:10.1111/dth.15285. doi:10.1111/jdv.12751.
Please refer to the full Cosentyx Prescribing Information here
Please refer to the full Cosentyx Consumer Medicine Information here
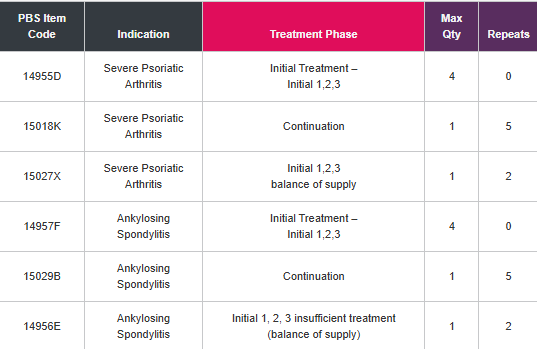
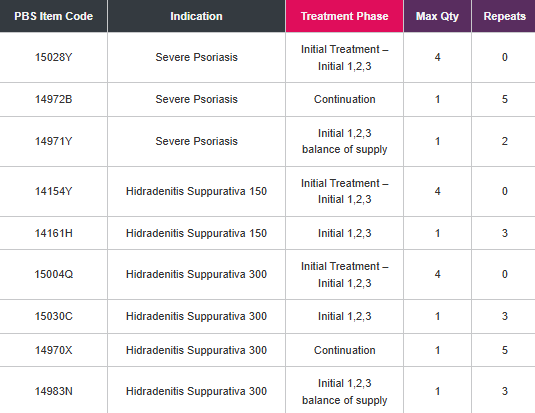
PBS Information: Section 85 Authority Required for the treatment of severe chronic plaque psoriasis, active ankylosing spondylitis, severe
psoriatic arthritis, non-radiographic axial spondyloarthritis and hidradenitis suppurativa. Refer to PBS Schedule for full Authority information.
▼This medicinal product is subject to additional monitoring in Australia due to approval of an extension of indications. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.



